Mesoporous silica is a form of silica that is characterised by its mesoporous structure, that is, having pores that range from 2 nm to 50 nm in diameter. According to IUPAC's terminology, mesoporosity sits between microporous (<2 nm) and macroporous (>50 nm). Mesoporous silica is a relatively recent development in nanotechnology. The most common types of mesoporous nanoparticles are MCM-41 and SBA-15.[2] Research continues on the particles, which have applications in catalysis, drug delivery and imaging.[3] Mesoporous ordered silica films have been also obtained with different pore topologies.[4]
A compound producing mesoporous silica was patented around 1970.[5][6][7] It went almost unnoticed[8] and was reproduced in 1997.[9] Mesoporous silica nanoparticles (MSNs) were independently synthesized in 1990 by researchers in Japan.[10] They were later produced also at Mobil Corporation laboratories[11] and named Mobil Composition of Matter (or Mobil Crystalline Materials, MCM).[12]
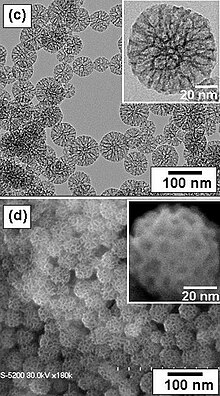
Six years later, silica nanoparticles with much larger (4.6 to 30 nanometer) pores were produced at the University of California, Santa Barbara.[13] The material was named Santa Barbara Amorphous type material, or SBA-15. These particles also have a hexagonal array of pores.
The researchers who invented these types of particles planned to use them as molecular sieves. Today, mesoporous silica nanoparticles have many applications in medicine, biosensors,[14] thermal energy storage,[15] water/gas filtration [16] and imaging.
- ^ Cite error: The named reference
A.B.D. Nandiyanto; S.-G Kim; F. Iskandar; and K. Okuyama 2009 447–453was invoked but never defined (see the help page). - ^ Katiyar, Amit; Yadav, Santosh; Smirniotis, Panagiotis G.; Pinto, Neville G. (July 2006). "Synthesis of ordered large pore SBA-15 spherical particles for adsorption of biomolecules". Journal of Chromatography A. 1122 (1–2): 13–20. doi:10.1016/j.chroma.2006.04.055. ISSN 0021-9673. PMID 16716334.
- ^ Trewyn, Brian G; Nieweg, Jennifer A; Zhao, Yannan; Lin, Victor S.-Y. (2007). "Biocompatible mesoporous silica nanoparticles with different morphologies for animal cell membrane penetration". Chemical Engineering Journal. 137 (1): 23–29. doi:10.1016/j.cej.2007.09.045.
- ^ Innocenzi, Plinio (2022). Mesoporous ordered silica films. From self-assembly to order. Advances in Sol-Gel Derived Materials and Technologies. Springer. doi:10.1007/978-3-030-89536-5. ISBN 978-3-030-89535-8. S2CID 245147740.
- ^ Chiola, V.; Ritsko, J. E. and Vanderpool, C. D. "Process for producing low-bulk density silica." Application No. US 3556725D A filed on 26-Feb-1969; Publication No. US 3556725 A published on 19-Jan-1971
- ^ "Porous silica particles containing a crystallized phase and method" Application No. US 3493341D A filed on 23-Jan-1967; Publication No. US 3493341 A published on 03-Feb-1970
- ^ "Process for producing silica in the form of hollow spheres"; Application No. US 342525 A filed on 04-Feb-1964; Publication No. US 3383172 A published on 14-May-1968
- ^ Xu, Ruren; Pang, Wenqin; Yu, Jihong (2007). Chemistry of zeolites and related porous materials: synthesis and structure. Wiley-Interscience. p. 472. ISBN 978-0-470-82233-3.
- ^ Direnzo, F; Cambon, H; Dutartre, R (1997). "A 28-year-old synthesis of micelle-templated mesoporous silica". Microporous Materials. 10 (4–6): 283–286. doi:10.1016/S0927-6513(97)00028-X.
- ^ Yanagisawa, Tsuneo; Shimizu, Toshio; Kuroda, Kazuyuki; Kato, Chuzo (1990). "The preparation of alkyltrimethylammonium-kanemite complexes and their conversion to microporous materials". Bulletin of the Chemical Society of Japan. 63 (4): 988–992. doi:10.1246/bcsj.63.988.
- ^ Beck, J. S.; Vartuli, J. C.; Roth, W. J.; Leonowicz, M. E.; Kresge, C. T.; Schmitt, K. D.; Chu, C. T. W.; Olson, D. H.; Sheppard, E. W. (1992). "A New Family of Mesoporous Molecular Sieves Prepared with Liquid Crystal Templates". Journal of the American Chemical Society. 114 (27): 10834–10843. doi:10.1021/ja00053a020.
- ^ Trewyn, B. G.; Slowing, I. I.; Giri, S; Chen, H. T.; Lin, V. S. (2007). "Synthesis and Functionalization of a Mesoporous Silica Nanoparticle Based on the Sol–Gel Process and Applications in Controlled Release". Accounts of Chemical Research. 40 (9): 846–853. doi:10.1021/ar600032u. PMID 17645305.
- ^ Zhao, Dongyuan; Feng, Jianglin; Huo, Qisheng; Melosh, Nicholas; Fredrickson, Glenn H.; Chmelka, Bradley F.; Stucky, Galen D. (1998). "Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores". Science. 279 (5350): 548–52. Bibcode:1998Sci...279..548Z. doi:10.1126/science.279.5350.548. PMID 9438845.
- ^ Valenti G, Rampazzo R, Bonacchi S, Petrizza L, Marcaccio M, Montalti M, Prodi L, Paolucci F (2016). "Variable Doping Induces Mechanism Swapping in Electrogenerated Chemiluminescence of Ru(bpy)32+ Core−Shell Silica Nanoparticles". J. Am. Chem. Soc. 138 (49): 15935–15942. doi:10.1021/jacs.6b08239. hdl:11585/583548. PMID 27960352.
- ^ Mitran, Raul−Augustin; Berger, Daniela; Munteanu, Cornel; Matei, Cristian (2015). "Evaluation of Different Mesoporous Silica Supports for Energy Storage in Shape-Stabilized Phase Change Materials with Dual Thermal Responses". The Journal of Physical Chemistry C. 119 (27): 15177–15184. doi:10.1021/acs.jpcc.5b02608.
- ^ Ghajeri, Farnaz; Topalian, Zareh; Tasca, Andrea; Jafri, Syed Hassan Mujtaba; Leifer, Klaus; Norberg, Peter; Sjöström, Christer (2018-08-01). "Case study of a green nanoporous material from synthesis to commercialisation: Quartzene®". Current Opinion in Green and Sustainable Chemistry. 12: 101–109. doi:10.1016/j.cogsc.2018.07.003. ISSN 2452-2236. S2CID 139146490.