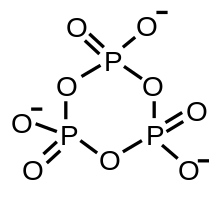
A metaphosphate ion is an oxyanion that has the empirical formula PO−
3.[1] It was first postulated in 1955[2] but was not observed until 1979, when it was detected by mass spectrometry.[3] Metaphosphate is an intermediate in the hydrolysis of phosphate esters but it is difficult to isolate, as it readily hydrolyses to from a dihydrogen phosphate ion ([H
2PO
4]−
) and tends to self-react in the absence of water to form rings or infinite chains:[4] These species are also called metaphosphates and are generally stable, with some such as sodium trimetaphosphate being produced on an industrial scale.
Metaphosphates can be used as an alternative of white phosphorus in organic syntheses.[5]
- ^ Averbuch-Pouchot, M.T; Durif, A. (1996). Topics in Phosphate Chemistry. World Scientific Pub Co Inc. ISBN 981-02-2634-9.
- ^ Butcher, W. W.; Westheimer, F. H. (May 1955). "The Lanthanum Hydroxide Gel Promoted Hydrolysis of Phosphate Esters". Journal of the American Chemical Society. 77 (9): 2420–2424. doi:10.1021/ja01614a018.
- ^ Harvan, Donald J.; Hass, J. Ronald; Busch, Kenneth L.; Bursey, Maurice M.; Ramirez, Fausto; Meyerson, Seymour (November 1979). "Direct observation of the monomeric metaphosphate anion". Journal of the American Chemical Society. 101 (24): 7409–7410. doi:10.1021/ja00518a050.
- ^ Regitz, Manfred; Maas, Gerhard (1981). "Short-lived phosphorus(V) compounds having coordination number 3". Organic Chemistry. 97: 71–120. doi:10.1007/BFb0037041.
- ^ "Sidestepping white phosphorus". C&EN Global Enterprise. 96 (7): 7. 2018. doi:10.1021/cen-09607-scicon1. ISSN 2474-7408.