| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Methylprop-2-enal | |||
| Other names
Methacrolein
Methacrylaldehyde Isobutenal | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.001.046 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
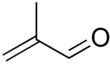
| C4H6O | |||
| Molar mass | 70.09 g/mol | ||
| Density | 0.847 g/cm3 | ||
| Melting point | −81 °C (−114 °F; 192 K) | ||
| Boiling point | 69 °C (156 °F; 342 K) | ||
| Related compounds | |||
Related alkenals
|
Citral | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Methacrolein, or methacrylaldehyde, is an unsaturated aldehyde. It is a clear, colorless, flammable liquid.
Methacrolein is one of two major products resulting from the reaction of isoprene with OH in the atmosphere, the other product being methyl vinyl ketone (MVK, also known as butenone).[1] These compounds are important components of the atmospheric oxidation chemistry of biogenic chemicals, which can result in the formation of ozone and/or particulates. Methacrylaldehyde is also present in cigarette smoke.[2] It can be found in the essential oil of the plant Big Sagebrush (Artemisia tridentata) which contains 5% methacrolein.[3]
Industrially, the primary use of methacrolein is in the manufacture of polymers and synthetic resins.
Exposure to methacrolein is highly irritating to the eyes, nose, throat and lungs.
- ^ Montzka, S. A.; Trainer, M.; Goldan, P. D.; Kuster, W. C.; Fehsenfeld, F. C. (1993). "Isoprene and its oxidation products, methyl vinyl ketone and methacrolein, in the rural troposphere". Journal of Geophysical Research: Atmospheres. 98 (D1): 1101–1111. Bibcode:1993JGR....98.1101M. doi:10.1029/92JD02382.
- ^ Roy J. Shephard (1982). The risks of passive smoking. ISBN 978-0-7099-2334-3. Retrieved 2009-05-06.
- ^ Shakhnoza, Azimova S.; et al. (2012). Lipids, Lipophilic Components and Essential Oils from Plant Sources. Springer. p. 844. ISBN 978-0-85729-323-7.