| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Methanesulfonic acid | |
| Other names
Methylsulfonic acid, MSA; Mesylic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1446024 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.817 |
| EC Number |
|
| 1681 | |
PubChem CID
|
|
| UNII | |
| UN number | 2585 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CH4O3S | |
| Molar mass | 96.10 g·mol−1 |
| Appearance | Clear, colourless liquid |
| Density | 1.48 g/cm3 |
| Melting point | 17 to 19 °C (63 to 66 °F; 290 to 292 K) |
| Boiling point | 167 °C (333 °F; 440 K) at 10 mmHg, 122 °C/1 mmHg |
| miscible | |
| Solubility | Miscible with methanol, diethyl ether. Immiscible with hexane |
| log P | −2.424[1] |
| Acidity (pKa) | −1.9[2] |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H314 | |
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |
| Safety data sheet (SDS) | Oxford MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
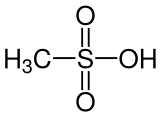
Methanesulfonic acid (MsOH, MSA) or methanesulphonic acid (in British English) is an organosulfuric, colorless liquid with the molecular formula CH3SO3H and structure H3C−S(=O)2−OH. It is the simplest of the alkylsulfonic acids (R−S(=O)2−OH). Salts and esters of methanesulfonic acid are known as mesylates (or methanesulfonates, as in ethyl methanesulfonate). It is hygroscopic in its concentrated form. Methanesulfonic acid can dissolve a wide range of metal salts, many of them in significantly higher concentrations than in hydrochloric acid (HCl) or sulfuric acid (H2SO4).[3]
- ^ Towler, Christopher S.; Li, Tonglei; Wikström, Håkan; Remick, David M.; Sanchez-Felix, Manuel V.; Taylor, Lynne S. (December 2008). "An Investigation into the Influence of Counterion on the Properties of Some Amorphous Organic Salts". Molecular Pharmaceutics. 5 (6): 946–955. doi:10.1021/mp8000342. PMID 19434850.
- ^ Guthrie, J. Peter (September 1978). "Hydrolysis of esters of oxy acids: pKa values for strong acids; Brønsted relationship for attack of water at methyl; free energies of hydrolysis of esters of oxy acids; and a linear relationship between free energy of hydrolysis and pKa holding over a range of 20 pK units". Canadian Journal of Chemistry. 56 (17): 2342–2354. doi:10.1139/v78-385.
- ^ Gernon, M. D.; Wu, M.; Buszta, T.; Janney, P. (1999). "Environmental benefits of methanesulfonic acid: comparative properties and advantages". Green Chemistry. 1 (3): 127–140. doi:10.1039/a900157c.