 | |
| Clinical data | |
|---|---|
| Other names | Deacetylsuperlutin; 16-Methylene-6-dehydro-17α-hydroxyprogesterone; 16-Methylenepregna-4,6-diene-3,20-dione; 16-Methyl-4,6,16-pregnatriene-3,20-dione |
| Drug class | Progestogen; Progestin |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C22H28O2 |
| Molar mass | 324.464 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
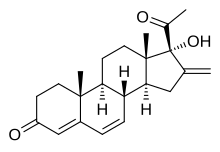
Methenmadinone, also known as deacetylsuperlutin or as 16-methylene-6-dehydro-17α-hydroxyprogesterone, is a pregnane steroid which was never marketed.[1][2][3] It is a parent compound of methenmadinone acetate (the C17α acetate ester), melengestrol (the C6 methyl derivative), and chlormethenmadinone (the C6 chloro derivative).[1][2]
- ^ a b G.W.A Milne (1 November 2017). Ashgate Handbook of Endocrine Agents and Steroids. Taylor & Francis. pp. 158–. ISBN 978-1-351-74347-1.
- ^ a b George W.A Milne (8 May 2018). Drugs: Synonyms and Properties: Synonyms and Properties. Taylor & Francis. pp. 1572–. ISBN 978-1-351-78989-9.
- ^ Shapiro EL, Weber L, Harris H, Miskowicz C, Neri R, Herzog HL (July 1972). "Synthesis and biological activity of 17-esters of 6-dehydro-16-methylene-17 -hydroxyprogesterones". J. Med. Chem. 15 (7): 716–20. doi:10.1021/jm00277a006. PMID 5043870.