| |

| |
| Names | |
|---|---|
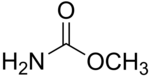
| Preferred IUPAC name
Methyl carbamate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.009.037 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H5NO2 | |
| Molar mass | 75 g/mol |
| Appearance | white solid |
| Density | 1.136 (56 °C) |
| Melting point | 52 °C (126 °F; 325 K) |
| Boiling point | 177 °C (351 °F; 450 K) |
| 20 g/L[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Methyl carbamate (also called methylurethane, or urethylane) is an organic compound and the simplest ester of carbamic acid (H2NCO2H). It is a colourless solid.[2]
Methyl carbamate is prepared by the reaction of methanol and urea:
- CO(NH2)2 + CH3OH → CH3OC(O)NH2 + NH3
It also forms in the reaction of ammonia with methyl chloroformate or dimethyl carbonate.
- ^ "Alfa Aesar Methyl carbamate". Alfa Aesar. Alfa Aesar. Retrieved 4 October 2021.
- ^ Jäger, Peter; Rentzea, Costin N.; Kieczka, Heinz (2012). "Carbamates and Carbamoyl Chlorides". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a05_051. ISBN 978-3527306732.