| |||
| Names | |||
|---|---|---|---|
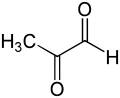
| Preferred IUPAC name
2-Oxopropanal | |||
| Other names
Pyruvaldehyde
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 906750 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.001.059 | ||
| KEGG | |||
| MeSH | Methylglyoxal | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H4O2 | |||
| Molar mass | 72.063 g·mol−1 | ||
| Appearance | Yellow liquid | ||
| Density | 1.046 g/cm3 | ||
| Melting point | 25 °C (77 °F; 298 K) | ||
| Boiling point | 72 °C (162 °F; 345 K) | ||
| Hazards | |||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H290, H302, H315, H317, H318, H319, H335, H341 | |||
| P201, P202, P234, P261, P264, P270, P271, P272, P280, P281, P301+P312, P302+P352, P304+P340, P305+P351+P338, P308+P313, P310, P312, P321, P330, P332+P313, P333+P313, P337+P313, P362, P363, P390, P403+P233, P404, P405, P501 | |||
| Related compounds | |||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Methylglyoxal (MGO) is the organic compound with the formula CH3C(O)CHO. It is a reduced derivative of pyruvic acid. It is a reactive compound that is implicated in the biology of diabetes. Methylglyoxal is produced industrially by degradation of carbohydrates using overexpressed methylglyoxal synthase.[1]
- ^ Lichtenthaler, Frieder W. (2010). "Carbohydrates as Organic Raw Materials". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.n05_n07. ISBN 978-3527306732.