 | |
| Clinical data | |
|---|---|
| Trade names | Relistor |
| Other names | MNTX, naltrexone-methyl-bromide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608052 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral, intravenous, subcutaneous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 11–15.3% |
| Metabolism | Liver |
| Elimination half-life | 8 hours |
| Excretion | Urine (50%), faeces (50%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.122.861 |
| Chemical and physical data | |
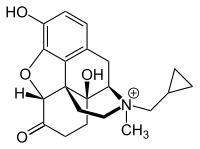
| Formula | C21H26NO4 |
| Molar mass | 356.442 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Methylnaltrexone (MNTX, brand name Relistor), used in form of methylnaltrexone bromide (INN, USAN, BAN), is a medication that acts as a peripherally acting μ-opioid receptor antagonist that acts to reverse some of the side effects of opioid drugs such as constipation without significantly affecting pain relief or precipitating withdrawals. Because MNTX is a quaternary ammonium cation, it cannot cross the blood–brain barrier, and so has antagonist effects throughout the body, counteracting effects such as itching and constipation, but without affecting opioid effects in the brain such as pain relief.[6] However, since a significant fraction (up to 60%) of opioid analgesia can be mediated by opioid receptors on peripheral sensory neurons, particularly in inflammatory conditions such as arthritis, traumatic or surgical pain,[7] MNTX may increase pain under such circumstances.
- ^ "Methylnaltrexone (Relistor) Use During Pregnancy". Drugs.com. 9 July 2020. Retrieved 21 September 2020.
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ "Relistor Product information". Health Canada. 25 April 2012. Retrieved 6 September 2022.
- ^ "Relistor Product information". Health Canada. 25 April 2012. Retrieved 6 September 2022.
- ^ "Relistor Product information". Health Canada. 25 April 2012. Retrieved 6 September 2022.
- ^ National Prescribing Service (1 March 2010). "Methylnaltrexone injections (Relistor) for opioid-induced constipation in palliative care". Archived from the original on 2010-06-01. Retrieved 12 March 2010.
- ^ Stein C (January 1993). "Peripheral mechanisms of opioid analgesia". Anesthesia and Analgesia. 76 (1): 182–91. doi:10.1213/00000539-199301000-00031. PMID 8380316. S2CID 10113604.