 | |
 | |
| Clinical data | |
|---|---|
| Other names | 3,4-Methylenedioxy-N-methylcathinone; Methylenedioxymethcathinone; MDMC; β-Keto-MDMA; βk-MDMA; M1; TSND-201; TSND201 |
| Routes of administration | Common: oral, insufflation Uncommon: IV or IM injection, rectal |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Onset of action | 0.5 hours[3] |
| Elimination half-life | 5.8–6.9 hours[3] |
| Duration of action | 2.5–3.0 hours[3] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
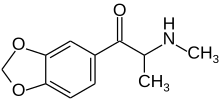
| Formula | C11H13NO3 |
| Molar mass | 207.229 g·mol−1 |
| 3D model (JSmol) | |
| Solubility in water | 357 mg/mL (20 °C) |
| |
| |
| | |
Methylone, also known as 3,4-methylenedioxy-N-methylcathinone (MDMC), is an empathogen and stimulant psychoactive drug. It is a member of the amphetamine, cathinone and methylenedioxyphenethylamine classes.
Methylone is a slight modification of 3,4-methylenedioxymethamphetamine (MDMA, also known as ecstasy). It was first synthesized by the chemists Peyton Jacob III and Alexander Shulgin in 1996 for potential use as an antidepressant.[4] Methylone has been sold for recreational use, taking advantage of the absence of legal prohibition of this compound in many countries.[citation needed]
- ^ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- ^ "Ustawa z dnia 15 kwietnia 2011 r. o zmianie ustawy o przeciwdziałaniu narkomanii ( Dz.U. 2011 nr 105 poz. 614 )". Internetowy System Aktów Prawnych. Retrieved 17 June 2011.
- ^ a b c Poyatos L, Lo Faro AF, Berardinelli D, Sprega G, Malaca S, Pichini S, et al. (November 2022). "Methylone and MDMA Pharmacokinetics Following Controlled Administration in Humans". International Journal of Molecular Sciences. 23 (23): 14636. doi:10.3390/ijms232314636. PMC 9736016. PMID 36498963.
- ^ WO 9639133, Jacob III P, Shulgin AT, "Novel N-Substituted-2-Amino-3',4'-Methylene-dioxypropiophenones", published 1996-12-12, assigned to Neurobiological Technologies Inc.