 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Zaroxolyn |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682345 |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~65% |
| Metabolism | kidney (minimal) |
| Elimination half-life | 14 hours |
| Excretion | primarily urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.037.748 |
| Chemical and physical data | |
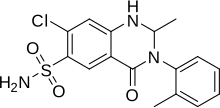
| Formula | C16H16ClN3O3S |
| Molar mass | 365.83 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 260 °C (500 °F) |
| |
| |
| | |
Metolazone is a thiazide-like diuretic marketed under the brand names Zytanix, Metoz, Zaroxolyn, and Mykrox. It is primarily used to treat congestive heart failure and high blood pressure. Metolazone indirectly decreases the amount of water reabsorbed into the bloodstream by the kidney, so that blood volume decreases and urine volume increases. This lowers blood pressure and prevents excess fluid accumulation in heart failure. Metolazone is sometimes used together with loop diuretics such as furosemide or bumetanide, but these highly effective combinations can lead to dehydration and electrolyte abnormalities.
It was patented in 1966 and approved for medical use in 1974.[1]
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 457. ISBN 9783527607495.