| Michael Addition | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reaction type | Addition reaction | ||||||||||
| Reaction | |||||||||||
| |||||||||||
| Identifiers | |||||||||||
| Organic Chemistry Portal | michael-addition | ||||||||||
| RSC ontology ID | RXNO:0000009 | ||||||||||
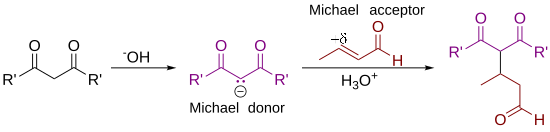
In organic chemistry, the Michael reaction or Michael 1,4 addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon.[1][2] It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds.[3]
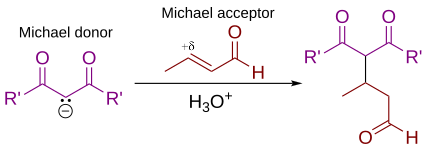
The Michael addition is an important atom-economical method for diastereoselective and enantioselective C–C bond formation, and many asymmetric variants exist[4][5][6]
In this general Michael addition scheme, either or both of R and R' on the nucleophile (the Michael donor) represent electron-withdrawing substituents such as acyl, cyano, nitro, or sulfone groups, which make the adjacent methylene hydrogen acidic enough to form a carbanion when reacted with the base, B:. For the alkene (the Michael acceptor), the R" substituent is usually a carbonyl, which makes the compound an α,β-unsaturated carbonyl compound (either an enone or an enal), or R" may be any electron withdrawing group.
- ^ Little, R. D.; Masjedizadeh, M. R.; Wallquist, O.; McLoughlin, J. I. (1995). "The Intramolecular Michael Reaction". Org. React. Vol. 47. pp. 315–552. doi:10.1002/0471264180.or047.02. ISBN 978-0-471-26418-7.
- ^ Mather, B.; Viswanathan, K.; Miller, K.; Long, T. (2006). "Michael addition reactions in macromolecular design for emerging technologies". Progress in Polymer Science. 31 (5): 487–531. doi:10.1016/j.progpolymsci.2006.03.001.
- ^ Michael Addition | PharmaXChange.info
- ^ Hunt, I. "Chapter 18: Enols and Enolates – The Michael Addition reaction". University of Calgary.
- ^ Clayden, Jonathan; Greeves, Nick; Warren, Stuart; Wothers, Peter (2001). Organic Chemistry (1st ed.). Oxford University Press. ISBN 978-0-19-850346-0.
- ^ Tiano, Martin (2020). "Enantioselective Michael Addition: An Experimental Introduction to Asymmetric Synthesis". Journal of Chemical Education. 97 (8): 2291–2295. Bibcode:2020JChEd..97.2291T. doi:10.1021/acs.jchemed.0c00164.