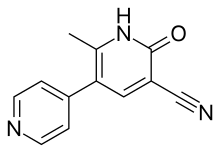
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601020 |
| Routes of administration | IV only |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% (as IV bolus, infusion) |
| Protein binding | 70 to 80% |
| Metabolism | Liver (12%) |
| Elimination half-life | 2.3 hours (mean, in CHF) |
| Excretion | Urine (85% as unchanged drug) within 24 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.071.709 |
| Chemical and physical data | |
| Formula | C12H9N3O |
| Molar mass | 211.224 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.344 g/cm3 |
| Melting point | 315 °C (599 °F) |
| |
| |
| (verify) | |
Milrinone, sold under the brand name Primacor, is a pulmonary vasodilator[2] used in patients who have heart failure. It is a phosphodiesterase 3 inhibitor that works to increase the heart's contractility and decrease pulmonary vascular resistance. Milrinone also works to vasodilate which helps alleviate increased pressures (afterload) on the heart, thus improving its pumping action. While it has been used in people with heart failure for many years, studies suggest that milrinone may exhibit some negative side effects that have caused some debate about its use clinically.[3][4]
Overall, milrinone supports ventricular functioning of the heart by decreasing the degradation of cyclic adenosine monophosphate (cAMP) and thus increasing phosphorylation levels of many components in the heart that contribute to contractility and heart rate. Milrinone is used as a drug that causes positive inotropy and it will lead to an increased force of contraction. Milrinone use following cardiac surgery has been under some debate because of the potential increase risk of postoperative atrial arrhythmias.[5] However, in the short term milrinone has been deemed beneficial to those experiencing heart failure and an effective therapy to maintain heart function following cardiac surgeries. There is no evidence of any long term beneficial effects on survival.[6] In critically ill patients with evidence of cardiac dysfunction there is limited good quality evidence to recommend its use.[7]
Milrinone is administered IV only and eliminated unchanged in the urine. Dose adjustment is required for patients with renal impairment.[8]
- ^ "Active substance: milrinone" (PDF). List of nationally authorised medicinal products. European Medicines Agency. 10 June 2022.
- ^ Baxter FJ, Whippey A (November 2020). "Amniotic Fluid Embolism Treated With Inhaled Milrinone: A Case Report". A&A Practice. 14 (13): e01342. doi:10.1213/XAA.0000000000001342. PMID 33185413. S2CID 226851766.
- ^ Packer M (December 1990). "Calcium channel blockers in chronic heart failure. The risks of "physiologically rational" therapy". Circulation. 82 (6): 2254–2257. doi:10.1161/01.cir.82.6.2254. PMID 2242549. S2CID 11255642.
- ^ Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, et al. (November 1991). "Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group". The New England Journal of Medicine. 325 (21): 1468–1475. doi:10.1056/NEJM199111213252103. PMID 1944425.
- ^ Fleming GA, Murray KT, Yu C, Byrne JG, Greelish JP, Petracek MR, et al. (October 2008). "Milrinone use is associated with postoperative atrial fibrillation after cardiac surgery". Circulation. 118 (16): 1619–1625. doi:10.1161/CIRCULATIONAHA.108.790162. PMC 2770257. PMID 18824641.
- ^ British National Formulary (66th ed.). London: BMJ Group and Pharmaceutical Press. September 2013.
- ^ Koster G, Bekema HJ, Wetterslev J, Gluud C, Keus F, van der Horst IC (September 2016). "Milrinone for cardiac dysfunction in critically ill adult patients: a systematic review of randomised clinical trials with meta-analysis and trial sequential analysis". Intensive Care Medicine. 42 (9): 1322–1335. doi:10.1007/s00134-016-4449-6. PMC 4992029. PMID 27448246.
- ^ "Milrinone Dosage Guide + Max Dose, Adjustments". Drugs.com. Retrieved 2023-01-03.