 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Minocin, Amzeeq, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682101 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous, topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 90–100%[4] |
| Protein binding | 70–75%[5] |
| Metabolism | Liver[5] |
| Elimination half-life | 14–22[5] (11–26[4]) hours |
| Excretion | Mostly fecal, 10–15% renal[5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.226.626 |
| Chemical and physical data | |
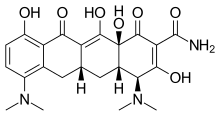
| Formula | C23H27N3O7 |
| Molar mass | 457.483 g·mol−1 |
| 3D model (JSmol) | |
| Specific rotation | = −166°[5] |
| Solubility in water | Low |
| |
| |
| | |
Minocycline, sold under the brand name Minocin among others, is a tetracycline antibiotic medication used to treat a number of bacterial infections such as some occurring in certain forms of pneumonia.[2][4][7] It is generally (but not always) less preferred than the tetracycline doxycycline.[4][7] Minocycline is also used for the treatment of acne and rheumatoid arthritis.[7][3] It is taken by mouth or applied to the skin.[4][3]
Common side effects include nausea, diarrhea, dizziness, allergic reactions, and kidney problems.[4] Serious side effects may include anaphylaxis, a lupus-like syndrome, and easy sunburning.[4] Use in the later part of pregnancy may harm the baby and safety during breastfeeding is unclear.[8] It works by decreasing a bacterium's ability to make protein thus stopping its growth.[4]
Minocycline was patented in 1961 and came into commercial use in 1971.[9] It is available as a generic medication.[7][10] In 2021, it was the 229th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[11][12]
- ^ "Minocycline Use During Pregnancy". Drugs.com. 4 December 2018. Retrieved 16 May 2020.
- ^ a b "Minocin- minocycline hydrochloride injection". DailyMed. 28 July 2021. Retrieved 19 February 2023.
- ^ a b c "Amzeeq- minocycline aerosol, foam". DailyMed. 25 January 2023. Retrieved 18 February 2023.
- ^ a b c d e f g h "Minocycline Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 23 March 2019.
- ^ a b c d e Dinnendahl V, Fricke U, eds. (2010). "Minocyclin". Arzneistoff-Profile (in German). Vol. 7 (24 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
- ^ "Minocycline". go.drugbank.com.
- ^ a b c d British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 556. ISBN 9780857113382.
- ^ "Minocycline Use During Pregnancy". Drugs.com. Retrieved 3 March 2019.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 489. ISBN 9783527607495.
- ^ "First Generic Drug Approvals". U.S. Food and Drug Administration. 17 October 2022. Retrieved 28 November 2022.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Minocycline - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.
![{\displaystyle [\alpha ]_{D}^{25}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/fc055b4d62c591651f8a4adbc6f6b2e9e71ce021)