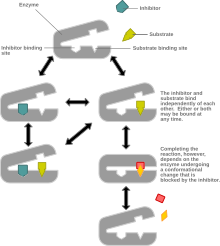

Mixed inhibition is a type of enzyme inhibition in which the inhibitor may bind to the enzyme whether or not the enzyme has already bound the substrate but has a greater affinity for one state or the other.[1] It is called "mixed" because it can be seen as a conceptual "mixture" of competitive inhibition, in which the inhibitor can only bind the enzyme if the substrate has not already bound, and uncompetitive inhibition, in which the inhibitor can only bind the enzyme if the substrate has already bound. If the ability of the inhibitor to bind the enzyme is exactly the same whether or not the enzyme has already bound the substrate, it is known as a non-competitive inhibitor.[1] [2] Non-competitive inhibition is sometimes thought of as a special case of mixed inhibition.
In mixed inhibition, the inhibitor binds to an allosteric site, i.e. a site different from the active site where the substrate binds. However, not all inhibitors that bind at allosteric sites are mixed inhibitors. [1]
Mixed inhibition may result in either:
- A decrease in the apparent affinity of the enzyme for the substrate (Km value appears to increase; ) -- seen in cases where the inhibitor favours binding to the free enzyme. More closely mimics competitive binding.
- An increase in the apparent affinity of the enzyme for the substrate (Km value appears to decrease; ) -- seen in cases where the inhibitor favours binding to the enzyme-substrate complex. More closely mimics uncompetitive binding.
In either case the inhibition decreases the apparent maximum enzyme reaction rate ().[3]
Mathematically, mixed inhibition occurs when the factors α and α’ (introduced into the Michaelis-Menten equation to account for competitive and uncompetitive inhibition, respectively) are both greater than 1.
In the special case where α = α’, noncompetitive inhibition occurs, in which case is reduced but is unaffected. This is very unusual in practice.[3]
- ^ a b c "Types of Inhibition". National Institutes of Health Chemical Genomics Center. 2011. Archived from the original on 8 September 2011. Retrieved 2 April 2012.
- ^ "Enzyme inhibition". London South Bank University. Archived from the original on 19 March 2012. Retrieved 2 April 2012.
- ^ a b Storey KB (2004). Functional Metabolism: Regulation and Adaptation. Wiley-IEEE. p. 12. ISBN 978-0-471-41090-4.




