 | |
| Clinical data | |
|---|---|
| Trade names | Univasc |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a695018 |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 13-22% |
| Protein binding | 90% |
| Metabolism | Hepatic (active metabolite, moexiprilat) |
| Elimination half-life | 1 hour; 2-9 hours (active metabolite) |
| Excretion | 50% (faeces), 13% (urine) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
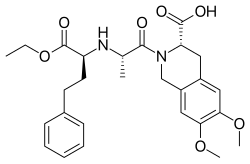
| Formula | C27H34N2O7 |
| Molar mass | 498.576 g·mol−1 |
| | |
Moexipril was an angiotensin converting enzyme inhibitor (ACE inhibitor)[1] used for the treatment of hypertension and congestive heart failure. Moexipril can be administered alone or with other antihypertensives or diuretics.[2]
It works by inhibiting the conversion of angiotensin I to angiotensin II.[3]
It was patented in 1980 and approved for medical use in 1995.[4] Moexipril is available from Schwarz Pharma under the trade name Univasc.[3][5]
- ^ Hochadel, Maryanne, ed. (2006). The AARP Guide to Pills. Sterling Publishing Company. p. 640. ISBN 978-1-4027-1740-6. Retrieved 2009-10-09.
- ^ Belal F, Metwaly FH, Younes KM, Amer SM (2009). "Development of Membrane Electrodes for the Specific Determination of Moexipril Hydrochloride in Dosage Forms and Biological Fluids". Portugaliae Electrochimica Acta. 27 (4): 463–475. doi:10.4152/pea.200904463.
- ^ a b Rodgers K, Vinson MC, Davis MW (1996). Breakthroughs: New drug approvals of 1995 -- part 1 (Report). Vol. 140. Advanstar Communications, Inc. p. 84.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 468. ISBN 9783527607495.
- ^ Banerji S (2004). "Angiotensin-Converting Enzyme Inhibitors". In Dart RC (ed.). Medical toxicology. Lippincott Williams & Wilkins. p. 647. ISBN 978-0-7817-2845-4. Retrieved 2009-10-09.