This article includes a list of general references, but it lacks sufficient corresponding inline citations. (March 2023) |
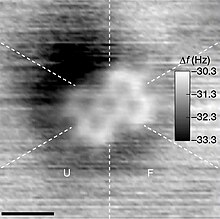


A molecule is a group of two or more atoms that are held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion.[4][5][6][7][8] In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.
A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term molecule is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms.[9] Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not considered single molecules.[10]
Concepts similar to molecules have been discussed since ancient times, but modern investigation into the nature of molecules and their bonds began in the 17th century. Refined over time by scientists such as Robert Boyle, Amedeo Avogadro, Jean Perrin, and Linus Pauling, the study of molecules is today known as molecular physics or molecular chemistry.
- ^ Iwata, Kota; Yamazaki, Shiro; Mutombo, Pingo; Hapala, Prokop; Ondráček, Martin; Jelínek, Pavel; Sugimoto, Yoshiaki (2015). "Chemical structure imaging of a single molecule by atomic force microscopy at room temperature". Nature Communications. 6: 7766. Bibcode:2015NatCo...6.7766I. doi:10.1038/ncomms8766. PMC 4518281. PMID 26178193.
- ^ Dinca, L.E.; De Marchi, F.; MacLeod, J.M.; Lipton-Duffin, J.; Gatti, R.; Ma, D.; Perepichka, D.F.; Rosei, F. (2015). "Pentacene on Ni(111): Room-temperature molecular packing and temperature-activated conversion to graphene". Nanoscale. 7 (7): 3263–9. Bibcode:2015Nanos...7.3263D. doi:10.1039/C4NR07057G. PMID 25619890.
- ^ Hapala, Prokop; Švec, Martin; Stetsovych, Oleksandr; Van Der Heijden, Nadine J.; Ondráček, Martin; Van Der Lit, Joost; Mutombo, Pingo; Swart, Ingmar; Jelínek, Pavel (2016). "Mapping the electrostatic force field of single molecules from high-resolution scanning probe images". Nature Communications. 7: 11560. Bibcode:2016NatCo...711560H. doi:10.1038/ncomms11560. PMC 4894979. PMID 27230940.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Molecule". doi:10.1351/goldbook.M04002
- ^ Ebbin, Darrell D. (1990). General Chemistry (3rd ed.). Boston: Houghton Mifflin Co. ISBN 978-0-395-43302-7.
- ^ Brown, T.L.; Kenneth C. Kemp; Theodore L. Brown; Harold Eugene LeMay; Bruce Edward Bursten (2003). Chemistry – the Central Science (9th ed.). New Jersey: Prentice Hall. ISBN 978-0-13-066997-1.
- ^ Chang, Raymond (1998). Chemistry (6th ed.). New York: McGraw Hill. ISBN 978-0-07-115221-1.
- ^ Zumdahl, Steven S. (1997). Chemistry (4th ed.). Boston: Houghton Mifflin. ISBN 978-0-669-41794-4.
- ^ Chandra, Sulekh (2005). Comprehensive Inorganic Chemistry. New Age Publishers. ISBN 978-81-224-1512-4.
- ^ "Molecule". Encyclopædia Britannica. 22 January 2016. Archived from the original on 3 May 2020. Retrieved 23 February 2016.