| |
| Names | |
|---|---|
| IUPAC name
Molybdenum tetrachloride
| |
| Other names
Molybdenum(IV) chloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.039 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| MoCl4 | |
| Molar mass | 237.752 g/mol |
| Appearance | black solid |
| Melting point | 552 °C (1,026 °F; 825 K) |
| Decomposes | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non flammable |
| Related compounds | |
Related compounds
|
Molybdenum(II) chloride Molybdenum(III) chloride Molybdenum(V) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
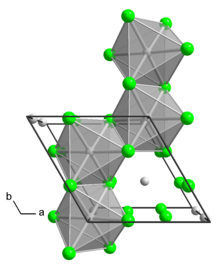
Molybdenum tetrachloride is the inorganic compound with the empirical formula MoCl4. The material exists as two polymorphs, both being dark-colored paramagnetic solids. These compounds are mainly of interest as precursors to other molybdenum complexes.
