| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Morpholine[2] | |||
| Other names
Diethylenimide oxide
1,4-Oxazinane Tetrahydro-1,4-oxazine Diethylene imidoxide Diethylene oximide Tetrahydro-p-oxazine | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 102549 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.469 | ||
| EC Number |
| ||
| 1803 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2054 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H9NO | |||
| Molar mass | 87.122 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Weak ammonia-like or fish-like[3] | ||
| Density | 1.007 g/cm3 | ||
| Melting point | −5 °C (23 °F; 268 K) | ||
| Boiling point | 129 °C (264 °F; 402 K) | ||
| miscible | |||
| Vapor pressure | 6 mmHg (20 °C)[3] | ||
| Acidity (pKa) | 8.36[4] (of conjugate acid) | ||
| -55.0·10−6 cm3/mol | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Flammable, Corrosive | ||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H226, H302, H312, H314, H332 | |||
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P322, P330, P363, P370+P378, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 31 °C (88 °F; 304 K) | ||
| 275 °C (527 °F; 548 K) | |||
| Explosive limits | 1.4%–11.2%[3] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
1220 mg/kg (mammal, oral) 525 mg/kg (mouse, oral) 1050 mg/kg (rat, oral)[5] | ||
LC50 (median concentration)
|
365 ppm (mouse, 2 hr)[5] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 20 ppm (70 mg/m3) [skin][3] | ||
REL (Recommended)
|
TWA 20 ppm (70 mg/m3) ST 30 ppm (105 mg/m3) [skin][3] | ||
IDLH (Immediate danger)
|
1400 ppm[3] | ||
| Safety data sheet (SDS) | hazard.com | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
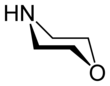
Morpholine is an organic chemical compound having the chemical formula O(CH2CH2)2NH. This heterocycle features both amine and ether functional groups. Because of the amine, morpholine is a base; its conjugate acid is called morpholinium. For example, treating morpholine with hydrochloric acid generates the salt morpholinium chloride. It is a colorless liquid with a weak, ammonia- or fish-like odor.[6] The naming of morpholine is attributed to Ludwig Knorr, who incorrectly believed it to be part of the structure of morphine.[7]
- ^ National Institute for Occupational Safety and Health (2000). "Morpholine". International Chemical Safety Cards. Retrieved 5 November 2005.
- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 142. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0437". National Institute for Occupational Safety and Health (NIOSH).
- ^ Hall, H. K. (1957). "Correlation of the Base Strengths of Amines1". J. Am. Chem. Soc. 79 (20): 5441–5444. doi:10.1021/ja01577a030.
- ^ a b "Morpholine". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "CDC - NIOSH Pocket Guide to Chemical Hazards - Morpholine". www.cdc.gov. Retrieved 4 January 2022.
- ^ F. Silversmith, Ernest; Nickon, Alex (2013-10-22). Organic Chemistry : Modern Coined Terms and Their Origins. Elsevier Science. p. 313. ISBN 978-1483145235.