 | |
| Clinical data | |
|---|---|
| Pronunciation | /mɒkˈsɒnɪdiːn/ |
| Trade names | Physiotens, Moxon |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral (tablets) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 88% (Tmax = 1 hour) |
| Protein binding | 7.2–10%[1][2] |
| Metabolism | Liver (10–20%)[2] |
| Metabolites | Dehydrogenated moxonidine (major), hydroxymethyl-moxonidine, hydroxy-moxonidine, dihydroxy-moxonidine[3] |
| Elimination half-life | ~2.2–2.8 hours |
| Excretion | Renal (90%),[4] feces (~1%)[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.158.061 |
| Chemical and physical data | |
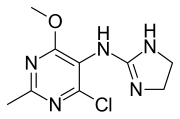
| Formula | C9H12ClN5O |
| Molar mass | 241.68 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Moxonidine (INN) is a new-generation alpha-2/imidazoline receptor agonist antihypertensive drug licensed for the treatment of mild to moderate essential hypertension.[5][6] It may have a role when thiazides, beta-blockers, ACE inhibitors, and calcium channel blockers are not appropriate or have failed to control blood pressure. In addition, it demonstrates favourable effects on parameters of the insulin resistance syndrome, apparently independent of blood pressure reduction. It is also a growth hormone releaser.[7] It is manufactured by Solvay Pharmaceuticals (acquired by Abbott in 2009) under the brand name Physiotens and Moxon.
- ^ Weimann HJ, Rudolph M (1992). "Clinical Pharmacokinetics of Moxonidine". Journal of Cardiovascular Pharmacology. 20 (Suppl. 4): S37–S41. doi:10.1097/00005344-199220004-00008.
- ^ a b c "Physiotens Tablets (moxonidine) Product Information" (PDF). Abbott Australasia Pty Ltd, 32-34 Lord Street, Botany NSW 2019, Australia. Retrieved 1 September 2016.
- ^ He MM, Abraham TL, Lindsay TJ, Schaefer HC, Pouliquen IJ, Payne C, et al. (March 2003). "Metabolism and disposition of the antihypertensive agent moxonidine in humans". Drug Metabolism and Disposition. 31 (3): 334–342. doi:10.1124/dmd.31.3.334. PMID 12584161.
- ^ Farsang, C (2001). "Moxonidine: Clinical Profile" (PDF). Journal of Clinical and Basic Cardiology. An Independent International Scientific Journal. 4 (3): 197–299. Retrieved 1 September 2016.
- ^ Fenton C, Keating GM, Lyseng-Williamson KA (2006). "Moxonidine: a review of its use in essential hypertension". Drugs. 66 (4): 477–496. doi:10.2165/00003495-200666040-00006. PMID 16597164. S2CID 195691757.
- ^ Fairbanks CA, Wilcox GL (July 1999). "Moxonidine, a selective alpha2-adrenergic and imidazoline receptor agonist, produces spinal antinociception in mice". The Journal of Pharmacology and Experimental Therapeutics. 290 (1): 403–412. PMID 10381806.
- ^ Bamberger CM, Mönig H, Mill G, Gödde E, Schulte HM (1995). "Growth hormone secretion in response to the new centrally acting antihypertensive agent moxonidine in normal human subjects: comparison to clonidine and GHRH". Experimental and Clinical Endocrinology & Diabetes. 103 (3): 205–208. doi:10.1055/s-0029-1211351. PMID 7584524.