| Murine respirovirus | |
|---|---|
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Negarnaviricota |
| Class: | Monjiviricetes |
| Order: | Mononegavirales |
| Family: | Paramyxoviridae |
| Genus: | Respirovirus |
| Species: | Murine respirovirus
|
| Synonyms | |
| |
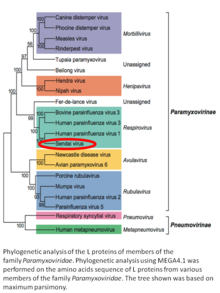
Murine respirovirus, formerly Sendai virus (SeV) and previously also known as murine parainfluenza virus type 1 or hemagglutinating virus of Japan (HVJ), is an enveloped, 150-200 nm–diameter, negative sense, single-stranded RNA virus of the family Paramyxoviridae.[2][3][4] It typically infects rodents and it is not pathogenic for humans or domestic animals.
Sendai virus (SeV) is a member of the genus Respirovirus.[5][6] The virus was isolated in the city of Sendai in Japan in the early 1950s. Since then, it has been actively used in research as a model pathogen. The virus is infectious for many cancer cell lines (see below), and has oncolytic properties demonstrated in animal models[7][8] and in naturally-occurring cancers in animals.[9] SeV's ability to fuse eukaryotic cells and to form syncytium was used to produce hybridoma cells capable of manufacturing monoclonal antibodies in large quantities.[10]
Recent applications of SeV-based vectors include the reprogramming of somatic cells into induced pluripotent stem cells[11][12] and vaccine creation. For vaccination purpose the Sendai virus-based constructs could be delivered in a form of nasal drops, which may be beneficial in inducing a mucosal immune response. SeV has several features that are important in a vector for a successful vaccine: the virus does not integrate into the host genome, it does not undergo genetic recombination, it replicates only in the cytoplasm without DNA intermediates or a nuclear phase and it does not cause any disease in humans or domestic animals. Sendai virus is used as a backbone for vaccine development against Mycobacterium tuberculosis that causes tuberculosis, against HIV-1 that causes AIDS and against other viruses, including those that cause severe respiratory infections in children.[13][14] The latter include Human Respiratory Syncytial Virus (HRSV), Human Metapneumovirus (HMPV) and Human Parainfluenza Viruses (HPIV).[14]
The vaccine studies against M. tuberculosis,[15] HMPV, HPIV1 and, HPIV2 are in the pre-clinical stage,[14] against HRSV a phase I clinical trail has been completed.[16] The phase I clinical studies of SeV-based vaccination were also completed for HPIV1.[14] They were done in adults and in 3- to 6-year-old children. As a result of vaccination against HPIV1 a significant boost in virus-specific neutralizing antibodies was observed.[14] A SeV-based vaccine development against HIV-1 has reached a phase II clinical trial.[17][18] In Japan intranasal Sendai virus-based SARS-CoV-2 vaccine was created and tested in a mouse model.[19]
- ^ Walker P (15 June 2015). "Implementation of taxon-wide non-Latinized binomial species names in the family Rhabdoviridae" (PDF). International Committee on Taxonomy of Viruses (ICTV). p. 7. Retrieved 6 February 2019.
- ^ Samal SK (2008). "Paramyxoviruses of Animals". Encyclopedia of Virology. Elsevier. pp. 40–47. doi:10.1016/b978-012374410-4.00460-x. ISBN 9780123744104. S2CID 81060576.
- ^ "Paramyxoviridae". UniProt.
- ^ Faísca P, Desmecht D (February 2007). "Sendai virus, the mouse parainfluenza type 1: a longstanding pathogen that remains up-to-date". Research in Veterinary Science. 82 (1): 115–125. doi:10.1016/j.rvsc.2006.03.009. PMID 16759680.
- ^ "Taxonomy - Respirovirus". UniProt.
- ^ "Respirovirus". ViralZone.
- ^ Cite error: The named reference
Saga_2015was invoked but never defined (see the help page). - ^ Cite error: The named reference
Matveeva 2015was invoked but never defined (see the help page). - ^ Cite error: The named reference
Ilyinskaya_2018was invoked but never defined (see the help page). - ^ Köhler G, Milstein C (August 1975). "Continuous cultures of fused cells secreting antibody of predefined specificity". Nature. 256 (5517): 495–497. Bibcode:1975Natur.256..495K. doi:10.1038/256495a0. PMID 1172191. S2CID 4161444.
- ^ Cite error: The named reference
Fusaki-2009was invoked but never defined (see the help page). - ^ Cite error: The named reference
Ban-2011was invoked but never defined (see the help page). - ^ Cite error: The named reference
Russell-2015was invoked but never defined (see the help page). - ^ a b c d e Russell CJ, Hurwitz JL (May 2021). "Sendai Virus-Vectored Vaccines That Express Envelope Glycoproteins of Respiratory Viruses". Viruses. 13 (6): 1023. doi:10.3390/v13061023. PMC 8230104. PMID 34072332.
- ^ Cite error: The named reference
Hu-2019was invoked but never defined (see the help page). - ^ Scaggs Huang F, Bernstein DI, Slobod KS, Portner A, Takimoto T, Russell CJ, et al. (February 2021). "Safety and immunogenicity of an intranasal sendai virus-based vaccine for human parainfluenza virus type I and respiratory syncytial virus (SeVRSV) in adults". Human Vaccines & Immunotherapeutics. 17 (2): 554–559. doi:10.1080/21645515.2020.1779517. PMC 7899675. PMID 32750273.
- ^ Cite error: The named reference
Seki-2016was invoked but never defined (see the help page). - ^ Cite error: The named reference
Nyombayire-2017was invoked but never defined (see the help page). - ^ Morimoto S, Saeki K, Takeshita M, Hirano K, Shirakawa M, Yamada Y, et al. (January 2023). "Intranasal Sendai virus-based SARS-CoV-2 vaccine using a mouse model". Genes to Cells. 28 (1): 29–41. doi:10.1111/gtc.12992. PMID 36401755. S2CID 253671438.