| |

| |
| Names | |
|---|---|
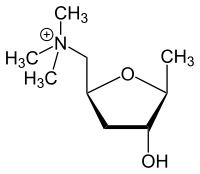
| IUPAC name
2,5-Anhydro-1,4,6-trideoxy-6-(trimethylazaniumyl)-D-ribo-hexitol
| |
| Systematic IUPAC name
1-[(2S,4R,5S)-4-Hydroxy-5-methyloxolan-2-yl]-N,N,N-trimethylmethanaminium | |
| Other names
L-(+)-muscarine, muscarin, (2S,4R,5S)-(4-hydroxy-5-methyl-tetrahydrofuran-2-ylmethyl)-trimethyl-ammonium
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.541 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H20NO2+ | |
| Molar mass | 174.26 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |

Muscarine, L-(+)-muscarine, or muscarin is a natural product found in certain mushrooms, particularly in Inocybe and Clitocybe species, such as the deadly C. dealbata. Mushrooms in the genera Entoloma and Mycena have also been found to contain levels of muscarine which can be dangerous if ingested. Muscarine has been found in harmless trace amounts in Boletus, Hygrocybe, Lactarius and Russula. Trace concentrations of muscarine are also found in Amanita muscaria, though the pharmacologically more relevant compound from this mushroom is the Z-drug-like alkaloid muscimol. A. muscaria fruitbodies contain a variable dose of muscarine, usually around 0.0003% fresh weight. This is very low and toxicity symptoms occur very rarely. Inocybe and Clitocybe contain muscarine concentrations up to 1.6%.[1]
Muscarine is a selective agonist of the muscarinic acetylcholine receptors.
- ^ Lurie, Y; Wasser, SP; Taha, M; Shehade, H; Nijim, J; Hoffmann, Y; Basis, F; Vardi, M; Lavon, O; Suaed, S; Bisharat, B; Bentur, Y (July 2009). "Mushroom poisoning from species of genus Inocybe (fiber head mushroom): a case series with exact species identification". Clinical Toxicology. 47 (6): 562–5. doi:10.1080/15563650903008448. PMID 19566380. S2CID 205902282.