| Myelin | |
|---|---|
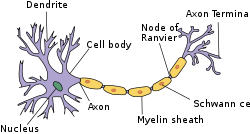 Structure of simplified neuron in the PNS | |
 Neuron with oligodendrocyte and myelin sheath in the CNS | |
| Details | |
| System | Nervous system |
| Identifiers | |
| FMA | 62977 |
| Anatomical terminology | |
Myelin (/ˈmaɪ.əlɪn/ MY-ə-lin) is a lipid-rich material that surrounds nerve cell axons (the nervous system's electrical wires) to insulate them and increase the rate at which electrical impulses (called action potentials) pass along the axon.[1][2] The myelinated axon can be likened to an electrical wire (the axon) with insulating material (myelin) around it. However, unlike the plastic covering on an electrical wire, myelin does not form a single long sheath over the entire length of the axon. Rather, myelin ensheaths the axon segmentally: in general, each axon is encased in multiple long sheaths with short gaps between, called nodes of Ranvier. At the nodes of Ranvier, which are approximately one thousandth of a mm (1 micron) in length, the axon's membrane is bare of myelin.
Myelin's best known function is to increase the rate at which information, encoded as electrical charges, passes along the axon's length. Myelin achieves this by eliciting saltatory conduction.[1] Saltatory conduction refers to the fact that electrical impulses 'jump' along the axon, over long myelin sheaths, from one node of Ranvier to the next. Thus, information is passed around 100 times faster along a myelinated axon than a non-myelinated one.
At the molecular level, the myelin sheath increases the distance between extracellular and intracellular ions, reducing the accumulation of electrical charges. The discontinuous structure of the myelin sheath results in the action potential "jumping" from one node of Ranvier over a long (c. 0.1 mm – >1 mm, or 100–1000 micron) myelinated stretch of the axon called the internodal segment or "internode", before "recharging" at the next node of Ranvier. This 'jumping' continues until the action potential reaches the axon terminal.[3][4][5] Once there, the electrical signal provokes the release of chemical neurotransmitters across the synapse, which bind to receptors on the post-synaptic cell (e.g. another neuron, myocyte or secretory cell).
Myelin is made by glial cells, which are non-neuronal cells that provide nutritional and homeostatic support to the axons. This is because axons, being elongated structures, are too far from the soma to be supported by the neurons themselves. In the central nervous system (brain, spinal cord and optic nerves), myelination is formed by specialized glial cells called oligodendrocytes, each of which sends out processes (limb-like extensions from the cell body) to myelinate multiple nearby axons; while in the peripheral nervous system, myelin is formed by neurolemmocytes (Schwann cells), which only myelinate a section of one axon. In the CNS, axons carry electrical signals from one nerve cell body to another.[6][7] The "insulating" function for myelin is essential for efficient motor function (i.e. movement such as walking), sensory function (e.g. sight, hearing, smell, the feeling of touch or pain) and cognition (e.g. acquiring and recalling knowledge), as demonstrated by the consequence of disorders that affect myelination, such as the genetically determined leukodystrophies;[8] the acquired inflammatory demyelinating disorder, multiple sclerosis;[9] and the inflammatory demyelinating peripheral neuropathies.[10] Due to its high prevalence, multiple sclerosis, which specifically affects the central nervous system (brain, spinal cord and optic nerve), is the best known disorder of myelin.
- ^ a b Bean, Bruce P. (June 2007). "The action potential in mammalian central neurons". Nature Reviews Neuroscience. 8 (6): 451–65. doi:10.1038/nrn2148. ISSN 1471-0048. PMID 17514198. S2CID 205503852.
- ^ Morell, Pierre; Quarles, Richard H. (1999). "The Myelin Sheath". Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition. Lippincott-Raven. Retrieved 15 December 2023.
- ^ Carroll, SL (2017). "The Molecular and Morphologic Structures That Make Saltatory Conduction Possible in Peripheral Nerve". Journal of Neuropathology and Experimental Neurology. 76 (4): 255–57. doi:10.1093/jnen/nlx013. PMID 28340093.
- ^ Keizer J, Smith GD, Ponce-Dawson S, Pearson JE (August 1998). "Saltatory propagation of Ca2+ waves by Ca2+ sparks". Biophysical Journal. 75 (2): 595–600. Bibcode:1998BpJ....75..595K. doi:10.1016/S0006-3495(98)77550-2. PMC 1299735. PMID 9675162.
- ^ Dawson SP, Keizer J, Pearson JE (May 1999). "Fire-diffuse-fire model of dynamics of intracellular calcium waves". Proceedings of the National Academy of Sciences of the United States of America. 96 (11): 6060–63. Bibcode:1999PNAS...96.6060D. doi:10.1073/pnas.96.11.6060. PMC 26835. PMID 10339541.
- ^ Stassart, Ruth M.; Möbius, Wiebke; Nave, Klaus-Armin; Edgar, Julia M. (2018). "The Axon-Myelin Unit in Development and Degenerative Disease". Frontiers in Neuroscience. 12: 467. doi:10.3389/fnins.2018.00467. ISSN 1662-4548. PMC 6050401. PMID 30050403.
- ^ Stadelmann, Christine; Timmler, Sebastian; Barrantes-Freer, Alonso; Simons, Mikael (2019-07-01). "Myelin in the Central Nervous System: Structure, Function, and Pathology". Physiological Reviews. 99 (3): 1381–431. doi:10.1152/physrev.00031.2018. ISSN 1522-1210. PMID 31066630.
- ^ van der Knaap MS, Bugiani M (September 2017). "Leukodystrophies: a proposed classification system based on pathological changes and pathogenetic mechanisms". Acta Neuropathologica. 134 (3): 351–82. doi:10.1007/s00401-017-1739-1. PMC 5563342. PMID 28638987.
- ^ Compston A, Coles A (October 2008). "Multiple sclerosis". Lancet. 372 (9648): 1502–17. doi:10.1016/S0140-6736(08)61620-7. PMID 18970977. S2CID 195686659.
- ^ Lewis RA (October 2017). "Chronic inflammatory demyelinating polyneuropathy". Current Opinion in Neurology. 30 (5): 508–12. doi:10.1097/WCO.0000000000000481. PMID 28763304. S2CID 4961339.