| |||

| |||
| |||
| Names | |||
|---|---|---|---|
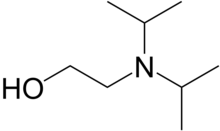
| Preferred IUPAC name
2-[Di(propan-2-yl)amino]ethan-1-ol | |||
| Other names
2-[Di(propan-2-yl)amino]ethanol
2-(Diisopropylamino)ethanol | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1697955 | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.307 | ||
| EC Number |
| ||
| MeSH | 2-diisopropylaminoethanol | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2922 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C8H19NO | |||
| Molar mass | 145.246 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Ammoniacal | ||
| Density | 826 mg mL−1 | ||
| Melting point | −39.2 °C; −38.6 °F; 233.9 K | ||
| Boiling point | 190.1 °C; 374.1 °F; 463.2 K | ||
| log P | 1.476 | ||
| Vapor pressure | <100 Pa (at 20 °C) | ||
Refractive index (nD)
|
1.442 | ||
| Hazards | |||
| GHS labelling: | |||
 
| |||
| H302, H311, H314, H331 | |||
| P261, P280, P305+P351+P338, P310 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 64 °C (147 °F; 337 K) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
| ||
| Safety data sheet (SDS) | [1] | ||
| Related compounds | |||
Related alkanols
|
|||
Related compounds
|
Diethylhydroxylamine | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
N,N-Diisopropylaminoethanol (DIPA) is a processor for production of various chemicals and also an intermediate in the production of the nerve agents VX and NX. [2] It is a colorless liquid, although aged samples can appear yellow.
- ^ Cite error: The named reference
sigmawas invoked but never defined (see the help page). - ^ Suzuki, Osamu; Kanako Watanabe, eds. (2005). Drugs and poisons in humans : a handbook of practical analysis (1. Aufl. ed.). Berlin [u.a.]: Springer. pp. 69–90. doi:10.1007/3-540-27579-7_9. ISBN 978-3-540-22277-4.


