This article needs more reliable medical references for verification or relies too heavily on primary sources. (June 2017) |  |
 | |
 | |
| Clinical data | |
|---|---|
| Other names | Dimethyltryptamine; DMT; N,N-DMT |
| Routes of administration | By mouth (usually with an MAOI), inhalation, insufflation, rectal, intramuscular, intravenous[1][2][3][4] |
| Drug class | Serotonergic psychedelic (hallucinogen)[1][2][4] |
| ATC code |
|
| Physiological data | |
| Source tissues | Central nervous system (exact source tissues are not fully established) |
| Target tissues | Central nervous system |
| Receptors | At least 13 receptors (e.g., serotonin, sigma, trace amine-associated) |
| Precursor | Tryptophan |
| Metabolism | Oxidative deamination (MAO-A), N-oxidation, N-demethylation, peroxidation[1][2] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Very low and inactive (except with an MAOI)[4] |
| Metabolism | Oxidative deamination (MAO-A), N-oxidation, N-demethylation, peroxidation[1][2] |
| Metabolites |
|
| Onset of action |
|
| Elimination half-life | |
| Duration of action |
|
| Excretion | Urine[4] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.463 |
| Chemical and physical data | |
| Formula | C12H16N2 |
| Molar mass | 188.274 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.099 g/cm3 |
| Melting point | 40 °C (104 °F) |
| Boiling point | 160 °C (320 °F) at 0.6 Torr (80 Pa)[7] also reported as 80–135 °C (176–275 °F) at 0.03 Torr (4.0 Pa)[8] |
| |
| |
| (verify) | |
| Part of a series on |
| Psychedelia |
|---|
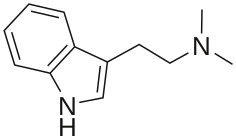 |
N,N-Dimethyltryptamine (DMT or N,N-DMT) is a substituted tryptamine that occurs in many plants and animals, including humans, and which is both a derivative and a structural analog of tryptamine.[1][2][3] DMT is used as a psychedelic drug and prepared by various cultures for ritual purposes as an entheogen.[9]
DMT has a rapid onset, intense effects, and a relatively short duration of action. For those reasons, DMT was known as the "businessman's trip" during the 1960s in the United States, as a user could access the full depth of a psychedelic experience in considerably less time than with other substances such as LSD or psilocybin mushrooms.[10] DMT can be inhaled, ingested, or injected and its effects depend on the dose, as well as the mode of administration. When inhaled or injected, the effects last about five to fifteen minutes. Effects can last three hours or more when orally ingested along with a monoamine oxidase inhibitor (MAOI), such as the ayahuasca brew of many native Amazonian tribes.[11] DMT can produce vivid "projections" of mystical experiences involving euphoria and dynamic pseudohallucinations of geometric forms.[12]
DMT is a functional analog and structural analog of other psychedelic tryptamines such as O-acetylpsilocin (4-AcO-DMT),[13] psilocybin (4-PO-DMT), psilocin (4-HO-DMT), NB-DMT, O-methylbufotenin (5-MeO-DMT), and bufotenin (5-HO-DMT). Parts of the structure of DMT occur within some important biomolecules like serotonin and melatonin, making them structural analogs of DMT.
- ^ a b c d e f g h i j k Cameron LP, Olson DE (October 2018). "Dark Classics in Chemical Neuroscience: N, N-Dimethyltryptamine (DMT)". ACS Chem Neurosci. 9 (10): 2344–2357. doi:10.1021/acschemneuro.8b00101. PMID 30036036.
- ^ a b c d e f g h i j k l Carbonaro TM, Gatch MB (September 2016). "Neuropharmacology of N,N-dimethyltryptamine". Brain Research Bulletin. 126 (Pt 1): 74–88. doi:10.1016/j.brainresbull.2016.04.016. PMC 5048497. PMID 27126737.
- ^ a b c d e f g h i Rodrigues AV, Almeida FJ, Vieira-Coelho MA (2019). "Dimethyltryptamine: Endogenous Role and Therapeutic Potential". J Psychoactive Drugs. 51 (4): 299–310. doi:10.1080/02791072.2019.1602291. hdl:10216/114373. PMID 31018803.
- ^ a b c d e f g h i j Cite error: The named reference
Brito-da-CostaDias-da-SilvaGomes2020was invoked but never defined (see the help page). - ^ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- ^ Barker SA (June 2022). "Administration of N,N-dimethyltryptamine (DMT) in psychedelic therapeutics and research and the study of endogenous DMT". Psychopharmacology (Berl). 239 (6): 1749–1763. doi:10.1007/s00213-022-06065-0. PMC 8782705. PMID 35064294.
- ^ Häfelinger G, Nimtz M, Horstmann V, Benz T (1999). "Untersuchungen zur Trifluoracetylierung der Methylderivate von Tryptamin und Serotonin mit verschiedenen Derivatisierungsreagentien: Synthesen, Spektroskopie sowie analytische Trennungen mittels Kapillar-GC" [Trifluoracetylation of methylated derivatives of tryptamine and serotonin by different reagents: synthesis, spectroscopic characterizations, and separations by capillary gas chromatography]. Zeitschrift für Naturforschung B. 54 (3): 397–414. doi:10.1515/znb-1999-0319. S2CID 101000504.
- ^ Corothie E, Nakano T (May 1969). "Constituents of the bark of Virola sebifera". Planta Medica. 17 (2): 184–188. doi:10.1055/s-0028-1099844. PMID 5792479. S2CID 43312376.
- ^ McKenna DJ, Towers GH, Abbott F (April 1984). "Monoamine oxidase inhibitors in South American hallucinogenic plants: tryptamine and beta-carboline constituents of ayahuasca". Journal of Ethnopharmacology. 10 (2): 195–223. doi:10.1016/0378-8741(84)90003-5. PMID 6587171.
- ^ Haroz R, Greenberg MI (November 2005). "Emerging drugs of abuse". The Medical Clinics of North America. 89 (6): 1259–1276. doi:10.1016/j.mcna.2005.06.008. OCLC 610327022. PMID 16227062.
- ^ Pickover C (2005). Sex, Drugs, Einstein, and Elves: Sushi, Psychedelics, Parallel Universes, and the Quest for Transcendence. Smart Publications. ISBN 978-1-890572-17-4.
- ^ "Erowid DMT (Dimethyltryptamine) Vault". Erowid.org. Archived from the original on 9 June 2022. Retrieved 20 September 2012.
- ^ Jones NT, Wagner L, Hahn MC, Scarlett CO, Wenthur CJ (2024-01-08). "In vivo validation of psilacetin as a prodrug yielding modestly lower peripheral psilocin exposure than psilocybin". Frontiers in Psychiatry. 14: 1303365. doi:10.3389/fpsyt.2023.1303365. PMC 10804612. PMID 38264637.