 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.020.031 |
| Chemical and physical data | |
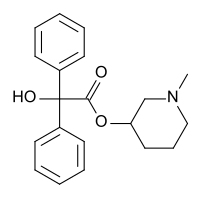
| Formula | C20H23NO3 |
| Molar mass | 325.408 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
N-Methyl-3-piperidyl benzilate (JB-336 or LBJ) is an anticholinergic drug related to the chemical warfare agent 3-quinuclidinyl benzilate.
N-methyl-3-piperidyl benzilate is less potent and shorter acting than 3-quinuclidyl benzilate, but like 3-QNB its effects on the central nervous system predominate over peripheral effects. It produces deliriant and hallucinogenic effects similar to those of plants such as datura and may be used recreationally at low doses; however, unpleasant side effects such as dysphoria, nausea and vomiting, dizziness and extreme dry mouth tend to make abuse of drugs of this kind uncommon. Both the N-methyl and N-ethyl analogues of 3-piperidyl benzilate are, however, Schedule I controlled drugs.
Radiolabelled versions of this drug are used in scientific research to map the distribution of muscarinic acetylcholine receptors in the brain.[1]
Methylation of JB-336 gives the quat salt, a Mepenzolate halide.
- ^ Takahashi K, Murakami M, Miura S, Iida H, Kanno I, Uemura K (March 1999). "Synthesis and autoradiographic localization of muscarinic cholinergic antagonist (+)N-[11C]methyl-3-piperidyl benzilate as a potent radioligand for positron emission tomography". Applied Radiation and Isotopes. 50 (3): 521–5. doi:10.1016/S0969-8043(97)10155-5. PMID 10070712.