 | |
| Clinical data | |
|---|---|
| Other names | mescaline-NBOMe; 345-NBOMe; N-(2-methoxybenzyl)-3,4,5-trimethoxyphenethylamine; 2-(3,4,5-trimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine; 3,4,5-Trimethoxy-N-(2-methoxybenzyl)phenethylamine |
| Routes of administration | Oral, intranasal, bucal, sublingual, intravenous |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | ? |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
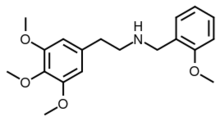
| Formula | C19H25NO4 |
| Molar mass | 331.412 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
NBOMe-mescaline or mescaline-NBOMe is a synthetic substituted phenethylamine. It is a partial agonist of serotonin receptors with a 5-HT2A pKi originally reported as 7.3 (i.e. Ki of approximately 50nM),[1] though more modern techniques assayed it as 140nM at 5-HT2A and 640nM at 5-HT2C, making it one of the least potent compounds among the N-benzyl phenethylamines.[2]
- ^ Cite error: The named reference
NS 1999was invoked but never defined (see the help page). - ^ Rickli A, Luethi D, Reinisch J, Buchy D, Hoener MC, Liechti ME (December 2015). "Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs)" (PDF). Neuropharmacology. 99: 546–53. doi:10.1016/j.neuropharm.2015.08.034. PMID 26318099. S2CID 10382311.