 | |
 | |
| Clinical data | |
|---|---|
| Trade names | nefopam medisol |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | intramuscular, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Low[1] |
| Protein binding | 70–75% (mean 73%)[1][2] |
| Metabolism | Liver (N-demethylation, others)[1] |
| Metabolites | Desmethylnefopam, others[1] |
| Elimination half-life | Nefopam: 3–8 hours[1] Desmethylnefopam: 10–15 hours[1] |
| Excretion | Urine: 79.3%[1] Feces: 13.4%[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.033.757 |
| Chemical and physical data | |
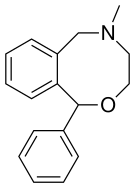
| Formula | C17H19NO |
| Molar mass | 253.345 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Nefopam, sold under the brand name Acupan among others, is a centrally acting, non-opioid painkilling medication, with central stimulant and sympathomimetic properties that is primarily used to treat moderate to severe pain.[3]
Nefopam acts in the brain and spinal cord to relieve pain via novel mechanisms: antinociceptive effects from triple monoamine reuptake inhibition, and antihyperalgesic activity through modulation of glutamatergic transmission.[4]
- ^ a b c d e f g h Sanga M, Banach J, Ledvina A, Modi NB, Mittur A (November 2016). "Pharmacokinetics, metabolism, and excretion of nefopam, a dual reuptake inhibitor in healthy male volunteers". Xenobiotica; the Fate of Foreign Compounds in Biological Systems. 46 (11): 1001–16. doi:10.3109/00498254.2015.1136989. PMID 26796604. S2CID 34603935.
- ^ Seyffart G (6 December 2012). Drug Dosage in Renal Insufficiency. Springer Science & Business Media. pp. 407–. ISBN 978-94-011-3804-8.
- ^ Brayfield A, ed. (27 October 2016). "Nefopam hydrochloride". MedicinesComplete. London, UK: Pharmaceutical Press. Retrieved 4 September 2017.
- ^ Cite error: The named reference
RevPharm2016was invoked but never defined (see the help page).