 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| Routes of administration | IV only |
| ATC code | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
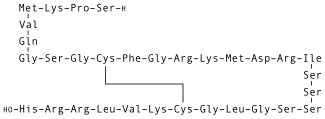
| Formula | C143H244N50O42S4 |
| Molar mass | 3464.07 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Nesiritide, sold under the brand name Natrecor, is the recombinant form of the 32 amino acid human B-type natriuretic peptide, which is normally produced by the ventricular myocardium. Nesiritide works to facilitate cardiovascular fluid homeostasis through counterregulation of the renin–angiotensin–aldosterone system, stimulating cyclic guanosine monophosphate, leading to smooth muscle cell relaxation.
Nesiritide was believed initially to be beneficial for acute decompensated congestive heart failure. It received approval from the United States' Food and Drug Administration for this purpose in 2001 after initial non-approval. In July 2011, the results of the largest study so far for nesiritide was published in The New England Journal of Medicine. The study failed to show a difference between nesiritide and placebo on mortality or re-hospitalizations.[1]
- ^ O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, et al. (July 2011). "Effect of nesiritide in patients with acute decompensated heart failure" (PDF). The New England Journal of Medicine. 365 (1): 32–43. doi:10.1056/NEJMoa1100171. hdl:11379/60663. PMID 21732835. Archived (PDF) from the original on 2021-02-11. Retrieved 2019-04-05.