This article is written like a research paper or scientific journal. (January 2023) |
| |
| Names | |
|---|---|
| Preferred IUPAC name
N,N′-Bis(4-nitrophenyl)urea—4,6-dimethylpyrimidin-2(1H)-one (1/1) | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.782 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H18N6O6 | |
| Molar mass | 426.38 g/mol |
| Appearance | light yellow powder |
| Density | 0.5 g/mL |
| Melting point | 265-275 C |
| slightly soluble in dimethylsulphoxide (DMSO) and dimethylformamide (DMF); insoluble in water and methanol | |
| Pharmacology | |
| QP51AE03 (WHO) | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration)
|
>0.147 mg/L in rats |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nicarbazin is a coccidiostat used on meat chickens. It is also used as a contraceptive for population control of Canada geese and feral pigeons.[1][2]
It is also a wide-spectrum anti-parasitic drug approved for veterinary use, effective on Toxocara canis, Toxascaris leonina, Ancylostoma caninum, Uncinaria stenocephala, Trichuris vulpis, Dipylidium caninum, and Taenia sp. and Mesocestoides sp.. Known brand names for specific countries include:[3]
- Carbigran (the United States)
- Ceva Nicarbazin (South Africa)
- Cycarb (New Zealand)
- Keymix (Australia)
- Koffogran (South Africa)
- Kofozin (Israel)
- Nicarb 25% (the United States)[4]
- Nicarbazin (Israel)
- Nicarbazin Elanco (the United States)
- Nicarbmax 100% (New Zealand)
- Nicarmix (the United States)
- Ovistop[5] (Italy [6] and Costa Rica)
- OvoControl (the United States)[6]
- PhiCarb (Australia)
- R-12[7] (Belgium)
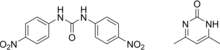
From the chemical point of view, nicarbazin is an equimolar complex formed by 1,3 - bis (4- nitrophenyl) urea and 4-6 dimethyl-2- (1H) - pyrimidinone also called 4,4'-dinitrocarbanilide (DNC) and 2-hydroxy - 4,6 dimethylpyrimidine (HDP).
The DNC represents the biologically active part of the complex, but to be absorbed it must be bound to the HDP.[8] Because of its hydrophobic nature, the DNC alone is poorly absorbed and has a limited "biological availability" so, following oral administration, it would be eliminated without being absorbed. The DNC therefore requires HDP to be absorbed and to reach a plasma level that allows an effect in the target species.
Following oral administration, nicarbazin rapidly dissociates in vivo into its two HDP and DNC components, which follow different routes of excretion: 95% of HDP is rapidly eliminated with urine, while DNC remains longer and is eliminated predominantly with feces.
Metabolism and depletion of the two components have been studied extensively with the use of carbon-14-labeled nicarbazin.[9] Following oral administration, nicarbazin rapidly dissociates in vivo into its two components HDP and DNC, which are absorbed through the intestine, then passing into the blood, and following different routes of excretion. The HDP is excreted more rapidly than the DNC, mainly through the kidneys via the urine, while the DNC is absorbed more rapidly than the HDP and is excreted more slowly than the latter through the liver, via the faeces.[10] No significant residue of either component is noticeable in any fabric after 7 days. DNC accumulates in eggs and normally the concentration of DNC in eggs is less than 5 ppm.
The two components HDP and DNC do not undergo metabolism in vivo and in vitro, except for the formation of derivatives structurally similar to nitroaniline. However, this possibility appears extremely remote, achievable only in the laboratory, in particular chemical-physical conditions (high temperature in a strongly acid environment).[11]
Of the two components of the nicarbazin molecule, the DNC takes on a significance from a toxicological point of view as it remains longer in the body. The DNC molecule was therefore considered as a marker compound in residue studies.
- ^ "US EPA - Nicarbazin Conditional Registration" (PDF). November 2005. Retrieved 27 August 2015.
- ^ Danaher, M.; Campbell, K.; O'Keeffe, M.; Capurro, E.; Kennedy, G.; Elliott, C. T. (2008). "Survey of the anticoccidial feed additive nicarbazin (as dinitrocarbanilide residues) in poultry and eggs" (PDF). Food Additives & Contaminants: Part A. 25 (1): 32–40. doi:10.1080/02652030701552956. PMID 17957540. S2CID 42464772.
- ^ "Wayback Machine has not archived that URL". Drugs.com. Retrieved 16 January 2023.[permanent dead link]
- ^ "Nicarb® 25% (Nicarbazin) TYPE A MEDICATED ARTICLE WITH MICROTRACER®". DailyMed. Retrieved 16 January 2023.
- ^ "www.ovistopinternational.com".[permanent dead link]
- ^ a b "The Efficacy of OvoControl® P (nicarbazin) in Feral Pigeons (Columba livia)" (PDF). 23 August 2010. Archived (PDF) from the original on 16 January 2022. Retrieved 16 January 2023.
- ^ "R-12".[permanent dead link]
- ^ Ott, WH, S. Kuna, CC Porter, and AC Cuckler. 1956. Biological studies on nicarbazin, a new anticoccidial agent. Poultry Science 35: 1355-1367.
- ^ World Health Organization (WHO), FAO Food and Nutrition Paper number 41/11. Residues of Some Veterinary Drugs in Animals and Foods (1999).
- ^ Porter CC, Gilfillan J. The absorption and excretion of orally administered nicarbazin in chickens Poultry Sci. 34, 995–1001, 1955
- ^ Valfrè F, Macrì A, "Nicarbazina: use in feeding the broilers and evaluation of residues" Veterinary Objectives 1990, 10: 1116