| |

| |
| Names | |
|---|---|
| Other names
bis[1,2-diphenyl-1,2-ethenedithiolato]nickel
bis(dithiobenzil)nickel(II) | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.044.853 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C28H20NiS4 | |
| Molar mass | 543.40 g·mol−1 |
| Appearance | black-green solid |
| Density | 1.466 g/cm3 |
| Melting point | 260 °C (500 °F; 533 K) |
| Structure[1] | |
| monoclinic | |
| P21/n | |
a = 0.5836 nm, b = 1.097 nm, c = 1.836 nm α = 90°, β = 91.4°, γ = 90°
| |
| Hazards | |
| GHS labelling: | |
| H317, H334, H350, H372 | |
| P260, P270, P272, P280 | |
| Safety data sheet (SDS) | Bis(dithiobenzil)nickel(II). TCI |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
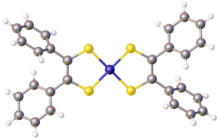
Nickel bis(stilbenedithiolate) or bis(dithiobenzil)nickel is a coordination complex with the formula Ni(S2C2Ph2)2 (where Ph = phenyl). It exists as a black solid that gives green solutions in toluene due to a strong absorption at 855 nm. The complex is a prototype of a large family of bis(dithiolene) complexes or the formula Ni(S2C2R2)2 (R = H, alkyl, aryl). These complexes have attracted much attention as dyes. They are of academic interest because the dithiolenes are noninnocent ligands.[2] The lengths of the C-S and C-C bonds in the backbone, respectively 1.71 and 1.39 Å, are intermediate between double and single bonds.[3]
The complex was prepared originally by treating nickel sulfide with diphenylacetylene.[4] High yielding syntheses involve treating nickel salts with sulfided benzoin. The complex reacts with ligands to form monodithiolene complexes of the type Ni(S2C2Ph2)L2.[5]
- ^ Sartain, D.; Truter, Mary R. (1967). "The crystal structure of bis(dithiobenzil)nickel". Journal of the Chemical Society A: Inorganic, Physical, Theoretical: 1264. doi:10.1039/J19670001264.
- ^ Karlin, K. D.; Stiefel, E. I., Eds. “Progress in Inorganic Chemistry, Dithiolene Chemistry: Synthesis, Properties, and Applications” Wiley-Interscience: New York, 2003. ISBN 0-471-37829-1
- ^ Miao, Qingqing; Gao, Junxiong; Wang, Zeqing; Yu, Hang; Luo, Yi; Ma, Tingli (2011). "Syntheses and Characterization of Several Nickel Bis(dithiolene) Complexes with Strong and Broad Near-IR Absorption". Inorganica Chimica Acta. 376: 619–627. doi:10.1016/j.ica.2011.07.046.
- ^ Schrauzer, G. N.; Mayweg, V. (1962). "Reaction of Diphenylacetylene with Nickel Sulfides". Journal of the American Chemical Society. 84 (16): 3221. doi:10.1021/ja00875a061.
- ^ Obanda, Antony; Martinez, Kristina; Schmehl, Russell H.; Mague, Joel T.; Rubtsov, Igor V.; MacMillan, Samantha N.; Lancaster, Kyle M.; Sproules, Stephen; Donahue, James P. (2017). "Expanding the Scope of Ligand Substitution from [M(S2C2Ph2] (M = Ni2+, Pd2+, Pt2+) to Afford New Heteroleptic Dithiolene Complexes" (PDF). Inorganic Chemistry. 56 (17): 10257–10267. doi:10.1021/acs.inorgchem.7b00971. PMID 28820242.
