| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Tetracarbonylnickel
| |||
| Other names
Nickel tetracarbonyl
Nickel carbonyl (1:4) Liquid Death | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 6122797 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.033.322 | ||
| EC Number |
| ||
| 3135 | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1259 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
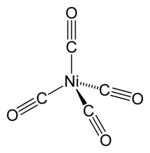
| Ni(CO)4 | |||
| Molar mass | 170.73 g/mol | ||
| Appearance | colorless liquid[1] | ||
| Odor | musty,[1] like brick dust | ||
| Density | 1.319 g/cm3 | ||
| Melting point | −17.2 °C (1.0 °F; 256.0 K) | ||
| Boiling point | 43 °C (109 °F; 316 K) | ||
| 0.018 g/100 mL (10 °C) | |||
| Solubility | miscible in most organic solvents soluble in nitric acid, aqua regia | ||
| Vapor pressure | 315 mmHg (20 °C)[1] | ||
| Viscosity | 3.05 x 10−4 Pa s | ||
| Structure | |||
| Tetrahedral | |||
| Tetrahedral | |||
| zero | |||
| Thermochemistry | |||
Std molar
entropy (S⦵298) |
320 J K−1 mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−632 kJ/mol | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−1180 kJ/mol | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Potential occupational carcinogen[2] | ||
| GHS labelling: | |||
   
| |||
| H225, H300, H301, H304, H310, H330, H351, H360D, H410 | |||
| P201, P202, P210, P233, P240, P241, P242, P243, P260, P271, P273, P280, P281, P284, P303+P361+P353, P304+P340, P308+P313, P310, P320, P370+P378, P391, P403+P233, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 4 °C (39 °F; 277 K) | ||
| 60 °C (140 °F; 333 K) | |||
| Explosive limits | 2–34% | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration)
|
266 ppm (cat, 30 min) 35 ppm (rabbit, 30 min) 94 ppm (mouse, 30 min) 10 ppm (mouse, 10 min)[3] | ||
LCLo (lowest published)
|
360 ppm (dog, 90 min) 30 ppm (human, 30 min) 42 ppm (rabbit, 30 min) 7 ppm (mouse, 30 min)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 0.001 ppm (0.007 mg/m3)[1] | ||
REL (Recommended)
|
TWA 0.001 ppm (0.007 mg/m3)[1] | ||
IDLH (Immediate danger)
|
Ca [2 ppm][1] | ||
| Safety data sheet (SDS) | ICSC 0064 | ||
| Related compounds | |||
Related metal carbonyls
|
| ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Nickel carbonyl (IUPAC name: tetracarbonylnickel) is a nickel(0) organometallic compound with the formula Ni(CO)4. This colorless liquid is the principal carbonyl of nickel. It is an intermediate in the Mond process for producing very high-purity nickel and a reagent in organometallic chemistry, although the Mond Process has fallen out of common usage due to the health hazards in working with the compound. Nickel carbonyl is one of the most dangerous substances yet encountered in nickel chemistry due to its very high toxicity, compounded with high volatility and rapid skin absorption.[4]
- ^ a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0444". National Institute for Occupational Safety and Health (NIOSH).
- ^ Nickel tetracarbonyl, carcinogenicity
- ^ a b "Nickel carbonyl". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ The Merck Index (7th ed.). Merck.


