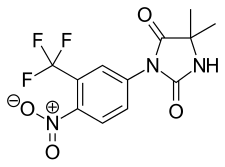
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | nye-LOO-tah-mide[1] |
| Trade names | Nilandron, Anandron |
| Other names | RU-23908 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697044 |
| Routes of administration | By mouth[2] |
| Drug class | Nonsteroidal antiandrogen |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Good[2] |
| Protein binding | 80–84%[4] |
| Metabolism | Liver (CYP2C19, FMO)[2][4] |
| Metabolites | At least 5, some active[4][5] |
| Elimination half-life | Mean: 56 hours (~2 days)[6] Range: 23–87 hours[6] |
| Excretion | Urine: 62%[2][4] Feces: <10%[2][4] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.153.268 |
| Chemical and physical data | |
| Formula | C12H10F3N3O4 |
| Molar mass | 317.224 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 149 °C (300 °F) |
| |
| |
| (verify) | |
Nilutamide, sold under the brand names Nilandron and Anandron, is a nonsteroidal antiandrogen (NSAA) which is used in the treatment of prostate cancer.[8][9][10][11][12][13] It has also been studied as a component of feminizing hormone therapy for transgender women and to treat acne and seborrhea in women.[14][15][16][17] It is taken by mouth.[4]
Side effects in men include breast tenderness and enlargement, feminization, sexual dysfunction, and hot flashes.[18][19][20][21] Nausea, vomiting, visual disturbances, alcohol intolerance, elevated liver enzymes, and lung disease can occur in both sexes.[21][22][19][23][24][25] Rarely, nilutamide can cause respiratory failure and liver damage.[18][21] These unfavorable side effects, along with a number of associated cases of death, have limited the use of nilutamide.[13][26][27]
Nilutamide acts as a selective antagonist of the androgen receptor (AR), preventing the effects of androgens like testosterone and dihydrotestosterone (DHT) in the body.[28][14] Because most prostate cancer cells rely on these hormones for growth and survival, nilutamide can slow the progression of prostate cancer and extend life in men with the disease.[14]
Nilutamide was discovered in 1977 and was first introduced for medical use in 1987.[9][29][30][6] It became available in the United States in 1996.[31][32][33] The drug has largely been replaced by newer and improved NSAAs, namely bicalutamide and enzalutamide, due to their better efficacy, tolerability, and safety, and is now rarely used.[34]
It is on the World Health Organization's List of Essential Medicines.[35]
- ^ Cite error: The named reference
LiverToxwas invoked but never defined (see the help page). - ^ a b c d e Perry MC, Doll DC, Freter CE (30 July 2012). Perry's The Chemotherapy Source Book. Lippincott Williams & Wilkins. pp. 711–. ISBN 978-1-4698-0343-2.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ a b c d e f Lemke TL, Williams DA (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1373–. ISBN 978-1-60913-345-0.
- ^ Cite error: The named reference
ChabnerLongo2010was invoked but never defined (see the help page). - ^ a b c Kolvenbag GJ, Furr BJ (2009). "Nonsteroidal Antiandrogens". In Jordan VC, Furr HJ (eds.). Hormone Therapy in Breast and Prostate Cancer. Humana Press. pp. 347–368. doi:10.1007/978-1-59259-152-7_16. ISBN 978-1-60761-471-5.
Although the t1/2 of nilutamide is h (mean 56 h) (39), suggesting that once-daily dosing would be appropriate, a three times per day regimen has been employed in most clinical trials.
- ^ "Nilutamide (Nilandron) Use During Pregnancy". Archived from the original on 28 October 2020. Retrieved 20 July 2016.
- ^ "NILANDRON® (nilutamide)" (PDF). Archived (PDF) from the original on 30 March 2021. Retrieved 25 September 2018.
- ^ a b Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 873–. ISBN 978-1-4757-2085-3.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 737–. ISBN 978-3-88763-075-1.
- ^ Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 199–. ISBN 978-94-011-4439-1.
- ^ "Nilutamide". Archived from the original on 2 December 2020. Retrieved 14 November 2017.
- ^ a b Denis LJ, Griffiths K, Kaisary AV, Murphy GP (1 March 1999). Textbook of Prostate Cancer: Pathology, Diagnosis and Treatment: Pathology, Diagnosis and Treatment. CRC Press. pp. 280–. ISBN 978-1-85317-422-3. Archived from the original on 10 January 2023. Retrieved 21 February 2016.
- ^ a b c Cite error: The named reference
Denis2012awas invoked but never defined (see the help page). - ^ Cite error: The named reference
KreukelsSteensma2013was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid2744186was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid2462132was invoked but never defined (see the help page). - ^ a b Dole EJ, Holdsworth MT (January 1997). "Nilutamide: an antiandrogen for the treatment of prostate cancer". The Annals of Pharmacotherapy. 31 (1): 65–75. doi:10.1177/106002809703100112. PMID 8997470. S2CID 20347526.
- ^ a b Cite error: The named reference
Dart2004was invoked but never defined (see the help page). - ^ Cite error: The named reference
MDMD2008was invoked but never defined (see the help page). - ^ a b c Cite error: The named reference
Lehne2013was invoked but never defined (see the help page). - ^ Cite error: The named reference
Becker2001was invoked but never defined (see the help page). - ^ Cite error: The named reference
WeinKavoussi2011was invoked but never defined (see the help page). - ^ Cite error: The named reference
Jafri2014was invoked but never defined (see the help page). - ^ Boarder MR, Newby D, Navti P (25 March 2010). Pharmacology for Pharmacy and the Health Sciences: A Patient-centred Approach. OUP Oxford. pp. 632–. ISBN 978-0-19-955982-4. Archived from the original on 6 July 2024. Retrieved 12 October 2016.
- ^ DeVita VT, Lawrence TS, Rosenberg SA, eds. (18 March 2016). Prostate and Other Genitourinary Cancers: Cancer: Principles & Practice of Oncology. Wolters Kluwer Health. pp. 1006–. ISBN 978-1-4963-5421-1.
- ^ Cite error: The named reference
Chang2005was invoked but never defined (see the help page). - ^ Cite error: The named reference
SinghGauthier2000was invoked but never defined (see the help page). - ^ Cite error: The named reference
LabrieLagacé1978was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid385986was invoked but never defined (see the help page). - ^ Cite error: The named reference
Pavlik2012was invoked but never defined (see the help page). - ^ Cite error: The named reference
BohlGao2005was invoked but never defined (see the help page). - ^ Cite error: The named reference
AdisInsightwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Gulley2011was invoked but never defined (see the help page). - ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.