| |
| Names | |
|---|---|
| Other names
niobium(II) oxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.031.631 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| NbO | |
| Molar mass | 108.905 g/mol[1] |
| Appearance | grey solid[1] |
| Odor | odorless |
| Density | 7.30 g/cm3[1] |
| Melting point | 1,937 °C (3,519 °F; 2,210 K)[1] |
| Solubility | slightly soluble in HCl insoluble in nitric acid |
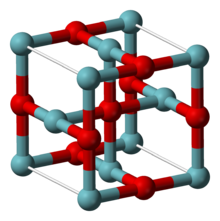
| Structure[2] | |
| Cubic, cP6 | |
| Pm3m, No. 221 | |
a = 0.4211 nm
| |
Formula units (Z)
|
3 |
| Thermochemistry[3] | |
Heat capacity (C)
|
41.3 J/(mol·K) |
Std molar
entropy (S⦵298) |
48.1 J/(mol·K) |
Std enthalpy of
formation (ΔfH⦵298) |
= -405.85 kJ/mol |
Gibbs free energy (ΔfG⦵)
|
-378.6 kJ/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Niobium monoxide is the inorganic compound with the formula NbO. It is a grey solid with metallic conductivity.[1]