 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Nityr, Orfadin |
| Other names | NTBC |
| AHFS/Drugs.com | Professional Drug Facts |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | Approximately 54 h |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.218.521 |
| Chemical and physical data | |
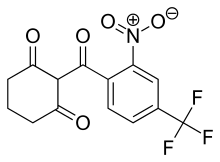
| Formula | C14H10F3NO5 |
| Molar mass | 329.231 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Nitisinone, sold under the brand name Orfadin among others, is a medication used to slow the effects of hereditary tyrosinemia type 1 (HT-1).
It is available as a generic medication.[2][3]
- ^ "Health Canada New Drug Authorizations: 2016 Highlights". Health Canada. 14 March 2017. Retrieved 7 April 2024.
- ^ "Competitive Generic Therapy Approvals". U.S. Food and Drug Administration (FDA). 29 June 2023. Archived from the original on 29 June 2023. Retrieved 29 June 2023.
- ^ "First Generic Drug Approvals 2023". U.S. Food and Drug Administration (FDA). 30 May 2023. Archived from the original on 30 June 2023. Retrieved 30 June 2023.