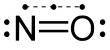| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Nitrogen monoxide[1]
| |||
| Systematic IUPAC name
Oxidonitrogen(•)[2] (additive) | |||
| Other names
Nitrogen oxide
Nitrogen(II) oxide Oxonitrogen Nitrogen monoxide | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.030.233 | ||
| EC Number |
| ||
| 451 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1660 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| NO | |||
| Molar mass | 30.006 g·mol−1 | ||
| Appearance | Colourless gas | ||
| Density | 1.3402 g/L | ||
| Melting point | −164 °C (−263 °F; 109 K) | ||
| Boiling point | −152 °C (−242 °F; 121 K) | ||
| 0.0098 g / 100 ml (0 °C) 0.0056 g / 100 ml (20 °C) | |||
Refractive index (nD)
|
1.0002697 | ||
| Structure | |||
| linear (point group C∞v) | |||
| Thermochemistry | |||
Std molar
entropy (S⦵298) |
210.76 J/(K·mol) | ||
Std enthalpy of
formation (ΔfH⦵298) |
90.29 kJ/mol | ||
| Pharmacology | |||
| R07AX01 (WHO) | |||
| License data | |||
| Inhalation | |||
| Pharmacokinetics: | |||
| good | |||
| via pulmonary capillary bed | |||
| 2–6 seconds | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Very toxic, corrosive, oxidizer[4] | ||
| GHS labelling: | |||
   [3][4] [3][4]
| |||
| Danger | |||
| H270, H314, H330[3][4] | |||
| P220, P244, P260, P280, P303+P361+P353+P315, P304+P340+P315, P305+P351+P338+P315, P370+P376, P403, P405[3][4] | |||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration)
|
315 ppm (rabbit, 15 min) 854 ppm (rat, 4 h) 2500 ppm (mouse, 12 min)[5] | ||
LCLo (lowest published)
|
320 ppm (mouse)[5] | ||
| Safety data sheet (SDS) | External SDS | ||
| Related compounds | |||
Related nitrogen oxides
|
Dinitrogen pentoxide Dinitrogen tetroxide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Nitric oxide (nitrogen oxide or nitrogen monoxide[1]) is a colorless gas with the formula NO. It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (•N=O or •NO). Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding.[6]
An important intermediate in industrial chemistry, nitric oxide forms in combustion systems and can be generated by lightning in thunderstorms. In mammals, including humans, nitric oxide is a signaling molecule in many physiological and pathological processes.[7] It was proclaimed the "Molecule of the Year" in 1992.[8] The 1998 Nobel Prize in Physiology or Medicine was awarded for discovering nitric oxide's role as a cardiovascular signalling molecule.[9] Its impact extends beyond biology, with applications in medicine, such as the development of sildenafil (Viagra), and in industry, including semiconductor manufacturing.[10][11]
Nitric oxide should not be confused with nitrogen dioxide (NO2), a brown gas and major air pollutant, or with nitrous oxide (N2O), an anesthetic gas.[6]
- ^ a b Nomenclature of Inorganic Chemistry, IUPAC Recommendations (PDF). International Union of Pure and Applied Chemistry. 2005. p. 69.
- ^ "Nitric Oxide (CHEBI:16480)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute.
- ^ a b c "Nitrogen monoxide - Registration Dossier - ECHA". Retrieved 2020-11-02.
- ^ a b c d "Safety Data Sheet - Nitric Oxide, compressed - Registration Dossier" (PDF). Retrieved 2020-11-02.
- ^ a b "Nitric oxide". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ a b Cite error: The named reference
G&Ewas invoked but never defined (see the help page). - ^ Hou, Y. C.; Janczuk, A.; Wang, P. G. (1999). "Current trends in the development of nitric oxide donors". Current Pharmaceutical Design. 5 (6): 417–441. doi:10.2174/138161280506230110111042. PMID 10390607.
- ^ Culotta, Elizabeth; Koshland, Daniel E. Jr. (1992). "NO news is good news". Science. 258 (5090): 1862–1864. Bibcode:1992Sci...258.1862C. doi:10.1126/science.1361684. PMID 1361684.
- ^ "The Nobel Prize in Physiology or Medicine 1998". NobelPrize.org. Retrieved 2022-06-17.
- ^ Reporter, Kashmira Gander (2020-04-07). "How the Gas That Gave Us Viagra Could Help Treat Coronavirus Patients". Newsweek. Retrieved 2024-08-29.
- ^ "Nitric Oxide in Semiconductor Manufacturing: Unveiling the Silent Powerhouse Shaping Our Hi-Tech Future | Plasma Futures". Retrieved 2024-08-29.