| |||

| |||
| Names | |||
|---|---|---|---|
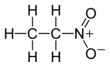
| Preferred IUPAC name
Nitroethane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.081 | ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | UN 2842 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H5NO2 | |||
| Molar mass | 75.067 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 1.054 g/cm3 | ||
| Melting point | −51 °C (−60 °F; 222 K) | ||
| Boiling point | 114 °C (237 °F; 387 K) | ||
| Slightly soluble (4.6 g/100 ml at 20 °C) | |||
| Vapor pressure | 21 mmHg (25 °C)[1] | ||
| Acidity (pKa) | 16.7[2][3] | ||
| -35.4·10−6 cm3/mol | |||
| Viscosity | 0.677 mPa·s at 20 °C | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Flammable, harmful | ||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H226, H302, H315, H331, H341, H412 | |||
| P210, P261, P301, P304, P312, P330, P340, P370, P378, P403+P233 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 28 °C (82 °F; 301 K) | ||
| Explosive limits | 3.4%-?[1] | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration)
|
5000 ppm (rabbit, 2 hr)[4] | ||
LCLo (lowest published)
|
6250 ppm (mouse, 2 hr)[4] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 100 ppm (310 mg/m3)[1] | ||
REL (Recommended)
|
TWA 100 ppm (310 mg/m3)[1] | ||
IDLH (Immediate danger)
|
1000 ppm[1] | ||
| Safety data sheet (SDS) | MSDS at fishersci.com | ||
| Related compounds | |||
Related nitro compounds
|
2-Nitropropane Nitromethane | ||
Related compounds
|
Ethyl nitrite Ethyl nitrate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Nitroethane is an organic compound having the chemical formula C2H5NO2. Similar in many regards to nitromethane, nitroethane is an oily liquid at standard temperature and pressure. Pure nitroethane is colorless and has a fruity odor.
- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0453". National Institute for Occupational Safety and Health (NIOSH).
- ^ Reich, Hans. "Bordwell pKa table: "Nitroalkanes"". University of Wisconsin Chemistry Department. Retrieved 17 January 2016.
- ^ Matthews, Walter; et al. (1975). "Equilibrium acidities of carbon acids. VI. Establishment of an absolute scale of acidities in dimethyl sulfoxide solution". Journal of the American Chemical Society. 97 (24): 7006. doi:10.1021/ja00857a010.
- ^ a b "Nitroethane". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).