| |
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
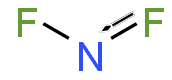
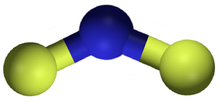
| NF2 | |
| Molar mass | 52.004 g·mol−1 |
| Related compounds | |
Related nitrogen fluorides
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nitrogen difluoride, also known as difluoroamino, is a reactive radical molecule with formula NF2. This small molecule is in equilibrium with its dimer tetrafluorohydrazine.[2]
- N2F4 ⇌ 2 NF2
As the temperature increases the proportion of NF2 increases.[3]
The molecule is unusual in that it has an odd number of electrons, yet is stable enough to study experimentally.[4]
- ^ Cite error: The named reference
trainor89was invoked but never defined (see the help page). - ^ Jäger, Susanne; von Jouanne, Jörn; Keller-Rudek, Hannelore; Koschel, Dieter; Kuhn, Peter; Merlet, Peter; Rupecht, Sigrid; Vanecek, Hans; Wagner, Joachim (1986). Koschel, Dieter; Kuhn, Peter; Merlet, Peter; Ruprecht, Sigrid; Wagner, Joachim (eds.). F Fluorine: Compounds with Oxygen and Nitrogen. Gmelin Handbook of Inorganic Chemistry. Vol. 4. Berlin: Springer. p. 162. doi:10.1007/978-3-662-06339-2. ISBN 978-3-662-06341-5. Retrieved 29 August 2015.
- ^ Johnson, Frederic A.; Colburn, Charles B. (July 1961). "The Tetrafluorohydrazine-Difluoroamino Radical Equilibrium". Journal of the American Chemical Society. 83 (14): 3043–3047. doi:10.1021/ja01475a018.
- ^ Brown, R. D.; Burden, F. R.; Hart, B. T.; Williams, G. R. (1973). "The electronic structure of the NF2 radical". Theoretica Chimica Acta. 28 (4): 339–353. doi:10.1007/BF00529015. S2CID 100649705.