| |||
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
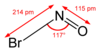
| NOBr | |||
| Molar mass | 109.910 g/mol | ||
| Appearance | Red gas | ||
| Boiling point | 14.5 °C (58.1 °F; 287.6 K) | ||
Refractive index (nD)
|
1.524 | ||
| Related compounds | |||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Nitrosyl bromide is the chemical compound with the chemical formula NOBr. It is a red gas with a condensing point just below room temperature.[1] It reacts with water.[1]
Nitrosyl bromide can be formed by the reversible reaction of nitric oxide with bromine.[2] This reaction is of interest as it is one of very few third-order homogeneous gas reactions. NOBr is prone to photodissociation at standard pressure and temperature.
- 2 NO + Br2 ⇌ 2 NOBr
Another way to make it is by way of nitric oxide reacting with potassium bromide.[1]
- 2NO2 + KBr → BrNO + KNO3
- ^ a b c Ratcliffe, Charles T.; Shreeve, Jean'ne M.; Wynne, Kenneth J. (January 1968). "Nitrosyl Halides". Inorganic Syntheses. Vol. 11. pp. 194–200. doi:10.1002/9780470132425.ch39. ISBN 9780470131701.
- ^ Esposti, C.D.; Tamassia, F.; Cazzoli, G.; Kisiel, Z. (April 1995). "Millimeter-Wave Spectrum of Nitrosyl Bromide in the Low-Lying Excited States: Equilibrium Structure and Cubic Force Field". Journal of Molecular Spectroscopy. 170 (2): 582–600. Bibcode:1995JMoSp.170..582E. doi:10.1006/jmsp.1995.1093.