 | |
| Clinical data | |
|---|---|
| Other names | 19-Normegestrol; 6-Methyl-17α-hydroxy-δ6-19-norprogesterone; 17α-Hydroxy-6-methyl-19-norpregna-4,6-diene-3,20-dione |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
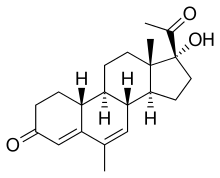
| Formula | C21H28O3 |
| Molar mass | 328.452 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Nomegestrol (INN), also known as 19-normegestrol, is a steroidal progestin which was patented in 1975 but was never marketed.[1][2] It is the parent compound of nomegestrol acetate, which is marketed as a progestin.[1][2]
Nomegestrol shows relatively low affinity for the progesterone receptor, only about 4% of that of progesterone and about 1.6% of that of nomegestrol acetate in one assay.[3]
- ^ a b J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 883–. ISBN 978-1-4757-2085-3.
- ^ a b Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 747–. ISBN 978-3-88763-075-1.
- ^ Botella J, Duc I, Delansorne R, Paris J, Lahlou B (December 1990). "Structure-activity and structure-affinity relationships of 19-nor-progesterone derivatives in rat uterus". J. Endocrinol. Invest. 13 (11): 905–10. doi:10.1007/BF03349652. PMID 2090671. S2CID 37429648.