This article may contain citations that do not verify the text. The reason given is: Checking of criteria section indicated that many were incorrect, so everything needs to be checked. (August 2024) |
| A periodic table extract highlighting nonmetals |
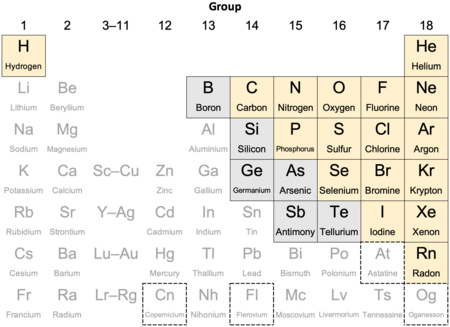
|
| always/usually considered nonmetals[1][2][3] |
| metalloids, sometimes considered nonmetals[a] |
| status as nonmetal or metal unconfirmed[5] |
| Part of a series on the |
| Periodic table |
|---|
In the context of the periodic table a nonmetal is a chemical element that mostly lacks distinctive metallic properties. They range from colorless gases like hydrogen to shiny crystals like iodine. Physically, they are usually lighter (less dense) than elements that form metals and are often poor conductors of heat and electricity. Chemically, nonmetals have relatively high electronegativity or usually attract electrons in a chemical bond with another element, and their oxides tend to be acidic.
Seventeen elements are widely recognized as nonmetals. Additionally, some or all of six borderline elements (metalloids) are sometimes counted as nonmetals.
The two lightest nonmetals, hydrogen and helium, together make up about 98% of the mass of the observable universe. Five nonmetallic elements—hydrogen, carbon, nitrogen, oxygen, and silicon—make up the bulk of Earth's atmosphere, biosphere, crust and oceans.
Industrial uses of nonmetals include in electronics, energy storage, agriculture, and chemical production.
Most nonmetallic elements were identified in the 18th and 19th centuries. While a distinction between metals and other minerals had existed since antiquity, a basic classification of chemical elements as metallic or nonmetallic emerged only in the late 18th century. Since then about twenty properties have been suggested as criteria for distinguishing nonmetals from metals.
- ^ Cite error: The named reference
Larrañagawas invoked but never defined (see the help page). - ^ Cite error: The named reference
Steudelwas invoked but never defined (see the help page). - ^ a b Cite error: The named reference
Vernon2013was invoked but never defined (see the help page). - ^ Cite error: The named reference
Goodrich etcwas invoked but never defined (see the help page). - ^ At: Restrepo et al. 2006, p. 411; Thornton & Burdette 2010, p. 86; Hermann, Hoffmann & Ashcroft 2013, pp. 11604‒1‒11604‒5; Cn: Mewes et al. 2019; Fl: Florez et al. 2022; Og: Smits et al. 2020
Cite error: There are <ref group=lower-alpha> tags or {{efn}} templates on this page, but the references will not show without a {{reflist|group=lower-alpha}} template or {{notelist}} template (see the help page).