 | |
| Clinical data | |
|---|---|
| Trade names | Vestalin (with EE) |
| Other names | Norvinodrel; Vinylestrenolone; Vinilestrenolone; Vinylnoretynodrel; 17α-Vinylestr-5(10)-en-17-ol-3-one; 17α-Vinyl-δ5(10)-19-nortestosterone |
| Routes of administration | By mouth |
| Drug class | Progestogen; Progestin |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
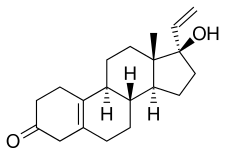
| Formula | C20H28O2 |
| Molar mass | 300.442 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Norgesterone, also known as norvinodrel or vinylestrenolone and sold under the brand name Vestalin, is a progestin medication which was formerly used in birth control pills for women but is now no longer marketed.[1][2][3][4] It was used in combination with the estrogen ethinylestradiol.[2][3][4] It is taken by mouth.[5][6]
Norgesterone is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[7] It has no androgenic activity.[7]
Norgesterone was first described in 1962.[8][9] It is no longer available.[10]
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 887–. ISBN 978-1-4757-2085-3.
- ^ a b Wassef SA, Sami G, Hamid EA (June 1970). "Effect of switching with oral contraceptives". The Egyptian Population and Family Planning Review. 3 (1): 77–93. PMID 12254511.
- ^ a b Bengtsson LP, Tausk M (September 1972). Pharmacology of the endocrine system and related drugs: progesterone, progestational drugs and antifertility agents. Pergamon Press. ISBN 9780080157450.
- ^ a b Challener CA (1 December 2001). Chiral Drugs. Wiley. ISBN 978-0-566-08411-9.
- ^ Boris Rubio L (November 1966). "[Vinylestrenolone: a new progestational hormone. Results of its cyclic administration]". Minerva Ginecologica (in Italian). 18 (21): 1215–1217. PMID 5997085.
- ^ Samaja BA, Prandini B (March 1974). "The influence of estrogenic and-or progestogenic treatment on some parameters of lipid metabolism (a controlled clinical study)". Endokrinologie. 63 (1): 76–84. PMID 4140086. Archived from the original on 2018-02-28.
- ^ a b de Ruggieri P, Matscher R, Lupo C, Spazzoli G (1965). "Biological properties of 17α-vinyl-5(10)-estrene-17β-ol-3-one (norvinodrel) as a progestational and claudogenic compound". Steroids. 5 (1): 73–91. doi:10.1016/0039-128X(65)90133-9. ISSN 0039-128X.
- ^ "Steroid hormone compositions and method of using same".
- ^ D'Incerti Bonini L, Pagani C (April 1962). "[Clinical investigations of the progestational activity of vinylestrenolone]". Annali di Ostetricia e Ginecologia (in Italian). 84: 279–285. PMID 13883015.
- ^ Cite error: The named reference
Micromedexwas invoked but never defined (see the help page).