| Nuclear physics |
|---|
 |
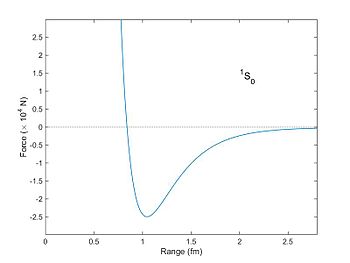
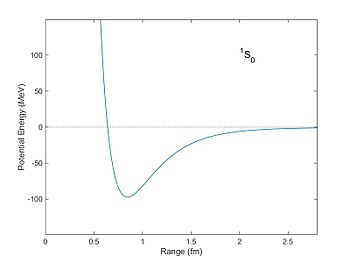
The nuclear force (or nucleon–nucleon interaction, residual strong force, or, historically, strong nuclear force) is a force that acts between hadrons, most commonly observed between protons and neutrons of atoms. Neutrons and protons, both nucleons, are affected by the nuclear force almost identically. Since protons have charge +1 e, they experience an electric force that tends to push them apart, but at short range the attractive nuclear force is strong enough to overcome the electrostatic force. The nuclear force binds nucleons into atomic nuclei.
The nuclear force is powerfully attractive between nucleons at distances of about 0.8 femtometre (fm, or 0.8×10−15 m), but it rapidly decreases to insignificance at distances beyond about 2.5 fm. At distances less than 0.7 fm, the nuclear force becomes repulsive. This repulsion is responsible for the size of nuclei, since nucleons can come no closer than the force allows. (The size of an atom, of size in the order of angstroms (Å, or 10−10 m), is five orders of magnitude larger.) The nuclear force is not simple, though, as it depends on the nucleon spins, has a tensor component, and may depend on the relative momentum of the nucleons.[2]
The nuclear force has an essential role in storing energy that is used in nuclear power and nuclear weapons. Work (energy) is required to bring charged protons together against their electric repulsion. This energy is stored when the protons and neutrons are bound together by the nuclear force to form a nucleus. The mass of a nucleus is less than the sum total of the individual masses of the protons and neutrons. The difference in masses is known as the mass defect, which can be expressed as an energy equivalent. Energy is released when a heavy nucleus breaks apart into two or more lighter nuclei. This energy is the internucleon potential energy that is released when the nuclear force no longer holds the charged nuclear fragments together.[3][4]
A quantitative description of the nuclear force relies on equations that are partly empirical. These equations model the internucleon potential energies, or potentials. (Generally, forces within a system of particles can be more simply modelled by describing the system's potential energy; the negative gradient of a potential is equal to the vector force.) The constants for the equations are phenomenological, that is, determined by fitting the equations to experimental data. The internucleon potentials attempt to describe the properties of nucleon–nucleon interaction. Once determined, any given potential can be used in, e.g., the Schrödinger equation to determine the quantum mechanical properties of the nucleon system.
The discovery of the neutron in 1932 revealed that atomic nuclei were made of protons and neutrons, held together by an attractive force. By 1935 the nuclear force was conceived to be transmitted by particles called mesons. This theoretical development included a description of the Yukawa potential, an early example of a nuclear potential. Pions, fulfilling the prediction, were discovered experimentally in 1947. By the 1970s, the quark model had been developed, by which the mesons and nucleons were viewed as composed of quarks and gluons. By this new model, the nuclear force, resulting from the exchange of mesons between neighbouring nucleons, is a multiparticle interaction, the collective effect of strong force on the underlining structure of the nucleons.
- ^ Reid, R. V. (1968). "Local phenomenological nucleon–nucleon potentials". Annals of Physics. 50 (3): 411–448. Bibcode:1968AnPhy..50..411R. doi:10.1016/0003-4916(68)90126-7.
- ^ Kenneth S. Krane (1988). Introductory Nuclear Physics. Wiley & Sons. ISBN 0-471-80553-X.
- ^ Binding Energy, Mass Defect Archived 2017-06-18 at the Wayback Machine, Furry Elephant physics educational site, retrieved 2012-07-01.
- ^ Chapter 4. NUCLEAR PROCESSES, THE STRONG FORCE Archived 2013-09-03 at the Wayback Machine, M. Ragheb, 1/30/2013, University of Illinois.