This article needs additional citations for verification. (March 2011) |
 | |
 | |
| Clinical data | |
|---|---|
| Other names | Psilacetin; 4-Acetoxy-N,N-dimethyltryptamine; 4-Acetoxy-DMT; 4-AcO-DMT; Synthetic shrooms; 3-(2'-Dimethylaminoethyl)-4-acetoxyindole[1] |
| Routes of administration | Oral, intravenous, intranasal, rectal |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
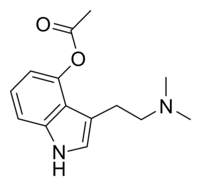
| Formula | C14H18N2O2 |
| Molar mass | 246.310 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 172 to 173 °C (342 to 343 °F) |
| |
| |
| (verify) | |
Psilacetin, also known as O-acetylpsilocin or as 4-acetoxy-N,N-dimethyltryptamine (4-acetoxy-DMT or 4-AcO-DMT), is a semi-synthetic serotonergic psychedelic drug that has been suggested by David Nichols to be a potentially useful alternative to psilocybin for pharmacological studies, as they are both believed to be prodrugs of psilocin.[2][3] However, some users report that O-acetylpsilocin's subjective effects differ from those of psilocybin and psilocin.[4][5] Additionally, some users prefer 4-AcO-DMT to natural psilocybin mushrooms due to feeling fewer adverse side effects such as nausea and heavy body load, which are more frequently reported in experiences involving natural mushrooms.[6] It is the acetylated form of the psilocybin mushroom alkaloid psilocin and is a lower homolog of 4-AcO-MET, 4-AcO-DET, 4-AcO-MiPT and 4-AcO-DiPT.
In 2024, it was confirmed that psilacetin is indeed a prodrug of psilocin.[7]
- ^ US patent 3075992, Hofmann A, Troxler F, "Esters of indoles", assigned to Sandoz Ltd.
- ^ Nichols D, Fescas S (1999). "Improvements to the Synthesis of Psilocybin and a Facile Method for Preparing the O-Acetyl Prodrug of Psilocin" (PDF). Synthesis. 1999 (6): 935–938. CiteSeerX 10.1.1.690.8071. doi:10.1055/s-1999-3490. S2CID 32044725. Archived (PDF) from the original on 17 February 2012. Retrieved 17 January 2012.
- ^ Bauer BE (2019-09-18). "The State of the Art of Psilacetin (4-AcO-DMT)". Psychedelic Science Review. Retrieved 2021-02-13.
- ^ "4-AcO-DMT (also 4-acetoxy-N,N-dimethyltryptamine) : Erowid Exp: Main Index". www.erowid.org. Archived from the original on 2010-07-28.
- ^ Janikian M (2020-05-26). "The Complete Guide: 4-AcO-DMT a.k.a. Synthetic Shrooms". DoubleBlind Mag.
- ^ Palamar JJ, Acosta P (January 2020). "A qualitative descriptive analysis of effects of psychedelic phenethylamines and tryptamines". Human Psychopharmacology. 35 (1): e2719. doi:10.1002/hup.2719. PMC 6995261. PMID 31909513.
- ^ Jones NT, Wagner L, Hahn MC, Scarlett CO, Wenthur CJ (2023). "In vivo validation of psilacetin as a prodrug yielding modestly lower peripheral psilocin exposure than psilocybin". Front Psychiatry. 14: 1303365. doi:10.3389/fpsyt.2023.1303365. PMC 10804612. PMID 38264637.