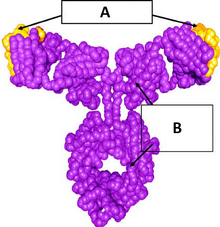
 Omalizumab structure: (A) murine complementarity-determining region and (B) IgG1κ human framework | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized (from mouse) |
| Target | IgE Fc region |
| Clinical data | |
| Pronunciation | /ˌoʊməˈlɪzumæb/ OH-mə-LI-zoo-mab |
| Trade names | Xolair |
| Biosimilars | Omlyclo[1][2] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603031 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Subcutaneous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 26 days |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C6450H9916N1714O2023S38 |
| Molar mass | 145058.53 g·mol−1 |
| | |
Omalizumab, sold under the brand name Xolair among others, is an injectable medication to treat severe persistent allergic forms of asthma, nasal polyps, urticaria (hives),[10][11] and immunoglobulin E-mediated food allergy.[12]
Omalizumab is a recombinant DNA-derived humanized IgG1 monoclonal antibody which specifically binds to free human immunoglobulin E (IgE) in the blood and interstitial fluid and to the membrane-bound form of IgE (mIgE) on the surface of mIgE-expressing B lymphocytes.[13][14] Its primary adverse effect is anaphylaxis.
In 1987, Tanox filed its first patent application on the anti-IgE drug candidates. It took until 2003 in the United States until omalizumab was approved, and in Europe until 2005 for moderate to severe persistent asthma and severe chronic rhinosinusitis with nasal polyps. In February 2024, the FDA approved it also to treat severe food allergy.
- ^ a b Cite error: The named reference
Omlyclo EPARwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Omlyclo EU PIwas invoked but never defined (see the help page). - ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Australian Public Assessment Report for Omalizumab" (PDF). Therapeutic Goods Administration. April 2021. Archived (PDF) from the original on 6 January 2023. Retrieved 5 January 2023.
- ^ "AusPAR Xolair Omalizumab Novartis Pharmaceuticals Australia Pty Ltd PM-2014-03868-1-5" (PDF). Therapeutic Goods Administration. 22 June 2016. Archived (PDF) from the original on 13 June 2021. Retrieved 5 January 2023.
- ^ "Regulatory Decision Summary - Xolair - Health Canada". Government of Canada. 14 July 2021. Archived from the original on 6 January 2023. Retrieved 5 January 2023.
- ^ Novartis Pharmaceuticals Canada Inc (26 September 2017). "Product Monograph: Pr Xolair Omalizumab" (PDF). Archived (PDF) from the original on 28 August 2021. Retrieved 5 January 2023.
- ^ "Regulatory Decision Summary for Xolair". Drug and Health Products Portal. 8 February 2024. Archived from the original on 2 April 2024. Retrieved 2 April 2024.
- ^ "Xolair 75 mg solution for injection in pre-filled syringe - Summary of Product Characteristics (SmPC)". (emc). 18 August 2020. Archived from the original on 13 June 2021. Retrieved 12 June 2021.
- ^ a b "Xolair- omalizumab injection, solution Xolair PFS- omalizumab injection, solution". DailyMed. 11 May 2020. Archived from the original on 28 November 2020. Retrieved 6 December 2020.
- ^ a b "Xolair EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 13 June 2021. Retrieved 12 June 2021.
- ^ Cite error: The named reference
FDA PR 20240216was invoked but never defined (see the help page). - ^ Presta LG, Lahr SJ, Shields RL, Porter JP, Gorman CM, Fendly BM, et al. (1993). "Humanization of an Antibody Directed Against IgE". The Journal of Immunology. 151 (5): 2623–2632. doi:10.4049/jimmunol.151.5.2623. PMID 8360482.
- ^ Schulman ES (October 2001). "Development of a monoclonal anti-immunoglobulin E antibody (omalizumab) for the treatment of allergic respiratory disorders". Am J Respir Crit Care Med. 164 (8 Pt 2): S6–11. doi:10.1164/ajrccm.164.supplement_1.2103025. PMID 11704611.