 | |
| Clinical data | |
|---|---|
| Trade names | Generic; many brand names[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682162 |
| Pregnancy category |
|
| Routes of administration | Oral, intravenous, intramuscular |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 90% |
| Protein binding | 95% |
| Metabolism | Hepatic demethylation |
| Elimination half-life | 13–20 hours[2] |
| Excretion | Renal and biliary |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.372 |
| Chemical and physical data | |
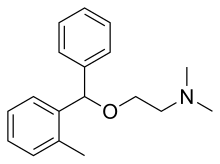
| Formula | C18H23NO |
| Molar mass | 269.388 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Orphenadrine (sold under many brand names)[1] is an anticholinergic drug of the ethanolamine antihistamine class; it is closely related to diphenhydramine. It is a muscle relaxant that is used to treat muscle pain and to help with motor control in Parkinson's disease, but has largely been superseded by newer drugs.[citation needed] It is considered a dirty drug due to its multiple mechanisms of action in different pathways.[citation needed] It was discovered and developed in the 1940s.
- ^ a b "Orphenadrine". Drugs.com international listings. Retrieved 5 February 2016.
- ^ Labout JJ, Thijssen C, Keijser GG, Hespe W (1982). "Difference between single and multiple dose pharmacokinetics of orphenadrine hydrochloride in man". European Journal of Clinical Pharmacology. 21 (4): 343–50. doi:10.1007/BF00637624. PMID 7056281. S2CID 24631265.