 | |
| Names | |
|---|---|
| IUPAC name
Silicic acid[1]
| |
| Other names
Orthosilicic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.030.421 |
| EC Number |
|
| 2009 | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
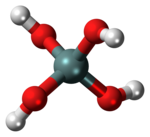
| Si(OH)4 | |
| Molar mass | 96.113 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Orthosilicic acid (/ˌɔːrθəsɪˈlɪsɪk/) is an inorganic compound with the formula Si(OH)4. Although rarely observed, it is the key compound of silica and silicates and the precursor to other silicic acids [H2xSiOx+2]n. Silicic acids play important roles in biomineralization and technology.[2][3][4] It is the parent acid of the orthosilicate anion SiO4−
4.
- ^ Nomenclature of inorganic chemistry: IUPAC recommendations 2005. Cambridge: Royal society of chemistry. 2005. p. 127. ISBN 0-85404-438-8.
- ^ N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997.
- ^ R. K. Iler, The Chemistry of Silica, Wiley, New York, 1979.
- ^ Gerhard Lagaly; Werner Tufar; A. Minihan; A. Lovell (2007). "Silicates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a23_661. ISBN 978-3527306732.