| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Oxalyl difluoride | |||
| Other names
TL-108
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.006.029 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
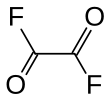
| C2F2O2 | |||
| Molar mass | 94.017 g/mol | ||
| Melting point | −3 °C (27 °F; 270 K) | ||
| Boiling point | 26.6 °C (79.9 °F; 299.8 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Oxalyl fluoride is the organofluorine compound with the formula (COF)2. It is a fluorinated derivative of oxalic acid. This colorless liquid is prepared by reaction of sodium fluoride with oxalyl chloride.[1]
Oxalyl fluoride is being investigated for use in etching as a replacement for compounds which have the liability of high global warming potential.[2][3]
- ^ C. W. Tullock, D. D. Coffman (1960). "Synthesis of Fluorides by Metathesis with Sodium Fluoride". J. Org. Chem. 25 (11): 2016–2019. doi:10.1021/jo01081a050.
- ^ Method of etching and cleaning using fluorinated carbonyl compounds, US Patent 6635185.
- ^ Simon Karecki; Ritwik Chatterjee; Laura Pruette; Rafael Reif; Terry Sparks; Laurie Beu; Victor Vartanian & Konstantin Novoselovc (2001). "Evaluation of Oxalyl Fluoride for a Dielectric Etch Application in an Inductively Coupled Plasma Etch Tool". J. Electrochem. Soc. 148 (3): G141–G149. Bibcode:2001JElS..148G.141K. doi:10.1149/1.1348263.