
| |
| |
| Names | |
|---|---|
| IUPAC name
Oxygen difluoride
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.029.087 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| OF2 | |
| Molar mass | 53.9962 g/mol |
| Appearance | colorless gas, pale yellow liquid when condensed |
| Odor | peculiar, foul |
| Density |
|
| Melting point | −223.8 °C (−370.8 °F; 49.3 K) |
| Boiling point | −144.75 °C (−228.55 °F; 128.40 K) |
| hydrolyzes[1] slowly | |
| Vapor pressure | 48.9 atm (at −58.0 °C or −72.4 °F or 215.2 K[a]) |
| Thermochemistry | |
Heat capacity (C)
|
43.3 J/mol K |
Std molar
entropy (S⦵298) |
246.98 J/mol K |
Std enthalpy of
formation (ΔfH⦵298) |
−24.5 kJ mol−1 |
Gibbs free energy (ΔfG⦵)
|
42.5 kJ/mol |
| Hazards | |
| GHS labelling:[4] | |
  
| |
| Danger | |
| H270, H314, H330 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration)
|
|
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.05 ppm (0.1 mg/m3)[2] |
REL (Recommended)
|
C 0.05 ppm (0.1 mg/m3)[2] |
IDLH (Immediate danger)
|
0.5 ppm[2] |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
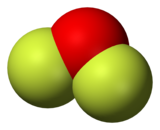
Oxygen difluoride is a chemical compound with the formula OF2. As predicted by VSEPR theory, the molecule adopts a bent molecular geometry. It is a strong oxidizer and has attracted attention in rocketry for this reason.[5] With a boiling point of −144.75 °C, OF2 is the most volatile (isolable) triatomic compound.[6] The compound is one of many known oxygen fluorides.
- ^ "difluorine monoxide; oxygen difluoride, physical properties, suppliers, CAS, MSDS, structure, Molecular Formula, Molecular Weight, Solubility, boiling point, melting point". www.chemyq.com.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0475". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Oxygen difluoride". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ GHS: GESTIS 570242
- ^ "Oxygen Difluoride - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 2023-11-03.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 819. ISBN 978-0-08-037941-8.
Cite error: There are <ref group=lower-alpha> tags or {{efn}} templates on this page, but the references will not show without a {{reflist|group=lower-alpha}} template or {{notelist}} template (see the help page).
