| PA clan of proteases | |
|---|---|
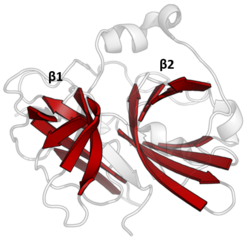 The double β-barrels that characterise the PA clan are highlighted in red. (TEV protease, PDB: 1lvm) | |
| Identifiers | |
| Symbol | N/A |
| Pfam clan | CL0124 |
| ECOD | 1.1.5 |
| InterPro | IPR009003 |
| SCOP2 | 50494 / SCOPe / SUPFAM |
| Membranome | 319 |
The PA clan (Proteases of mixed nucleophile, superfamily A) is the largest group of proteases with common ancestry as identified by structural homology. Members have a chymotrypsin-like fold and similar proteolysis mechanisms but can have identity of <10%. The clan contains both cysteine and serine proteases (different nucleophiles).[1][2] PA clan proteases can be found in plants,[3] animals,[3] fungi,[3] eubacteria,[4] archaea[5][6] and viruses.[2]
The common use of the catalytic triad for hydrolysis by multiple clans of proteases, including the PA clan, represents an example of convergent evolution.[7] The differences in the catalytic triad within the PA clan is also an example of divergent evolution of active sites in enzymes.[2]
- ^ Rawlings ND, Barrett AJ, Bateman A (January 2012). "MEROPS: the database of proteolytic enzymes, their substrates and inhibitors". Nucleic Acids Research. 40 (Database issue): D343-50. doi:10.1093/nar/gkr987. PMC 3245014. PMID 22086950.
- ^ a b c Bazan JF, Fletterick RJ (November 1988). "Viral cysteine proteases are homologous to the trypsin-like family of serine proteases: structural and functional implications". Proceedings of the National Academy of Sciences of the United States of America. 85 (21): 7872–6. Bibcode:1988PNAS...85.7872B. doi:10.1073/pnas.85.21.7872. PMC 282299. PMID 3186696.
- ^ a b c Laskar A, Rodger EJ, Chatterjee A, Mandal C (May 2012). "Modeling and structural analysis of PA clan serine proteases". BMC Research Notes. 5: 256. doi:10.1186/1756-0500-5-256. PMC 3434108. PMID 22624962.
- ^ Barbosa JA, Saldanha JW, Garratt RC (July 1996). "Novel features of serine protease active sites and specificity pockets: sequence analysis and modelling studies of glutamate-specific endopeptidases and epidermolytic toxins". Protein Engineering. 9 (7): 591–601. doi:10.1093/protein/9.7.591. PMID 8844831.
- ^ "MEROPS - Archaeal S01 proteases".[permanent dead link]
- ^ Ruiz-Perez F, Nataro JP (March 2014). "Bacterial serine proteases secreted by the autotransporter pathway: classification, specificity, and role in virulence". Cellular and Molecular Life Sciences. 71 (5): 745–70. doi:10.1007/s00018-013-1355-8. PMC 3871983. PMID 23689588.
- ^ Buller AR, Townsend CA (February 2013). "Intrinsic evolutionary constraints on protease structure, enzyme acylation, and the identity of the catalytic triad". Proceedings of the National Academy of Sciences of the United States of America. 110 (8): E653-61. Bibcode:2013PNAS..110E.653B. doi:10.1073/pnas.1221050110. PMC 3581919. PMID 23382230.