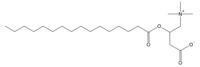
| |
| Names | |
|---|---|
| IUPAC name
3-(palmitoyloxy)-4-(trimethylammonio)butanoate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| MeSH | Palmitoylcarnitine |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C23H45NO4 | |
| Molar mass | 399.608 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Palmitoylcarnitine is an ester derivative of carnitine involved in the metabolism of fatty acids. During the tricarboxylic acid cycle (TCA), fatty acids undergo a process known as β-oxidation to produce energy in the form of ATP. β-oxidation occurs primarily within mitochondria, however the mitochondrial membrane prevents the entry of long chain fatty acids (>C10), so the conversion of fatty acids such as palmitic acid is key.[1] Palmitic acid is brought to the cell and once inside the cytoplasm is first converted to Palmitoyl-CoA. Palmitoyl-CoA has the ability to freely pass the outer mitochondrial membrane, but the inner membrane is impermeable to the Acyl-CoA and thioester forms of various long-chain fatty acids such as palmitic acid. The palmitoyl-CoA is then enzymatically transformed into palmitoylcarnitine via the Carnitine O-palmitoyltransferase family. The palmitoylcarnitine is then actively transferred into the inner membrane of the mitochondria via the carnitine-acylcarnitine translocase.[2] Once inside the inner mitochondrial membrane, the same Carnitine O-palmitoyltransferase family is then responsible for transforming the palmitoylcarnitine back to the palmitoyl-CoA form.
- ^ Tein, Ingrid (2015-01-01), Darras, Basil T.; Jones, H. Royden; Ryan, Monique M.; De Vivo, Darryl C. (eds.), "Chapter 40 - Lipid Storage Myopathies Due to Fatty Acid Oxidation Defects", Neuromuscular Disorders of Infancy, Childhood, and Adolescence (Second Edition), San Diego: Academic Press, pp. 761–795, doi:10.1016/b978-0-12-417044-5.00040-8, ISBN 978-0-12-417044-5
- ^ Pande, S. V. (1975-03-01). "A mitochondrial carnitine acylcarnitine translocase system". Proceedings of the National Academy of Sciences. 72 (3): 883–887. Bibcode:1975PNAS...72..883P. doi:10.1073/pnas.72.3.883. ISSN 0027-8424. PMC 432425. PMID 1055387.